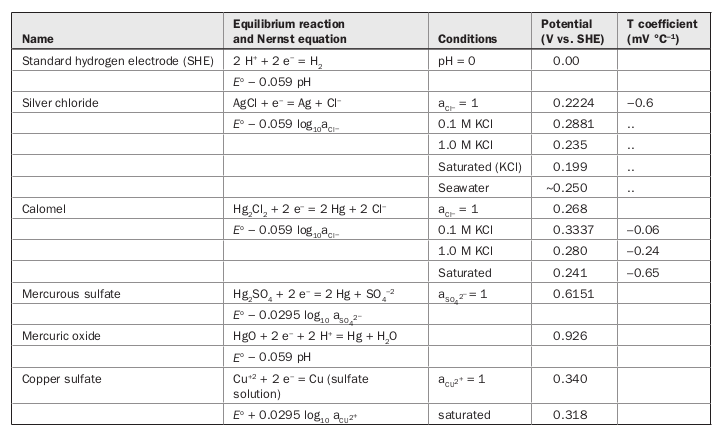
![PDF] A review of screen-printed silver/silver chloride (Ag/AgCl) reference electrodes potentially suitable for environmental potentiometric sensors | Semantic Scholar PDF] A review of screen-printed silver/silver chloride (Ag/AgCl) reference electrodes potentially suitable for environmental potentiometric sensors | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/8bb8cc3ee724ec4d616f3f6fad0294a6e0e36799/14-Table1-1.png)
PDF] A review of screen-printed silver/silver chloride (Ag/AgCl) reference electrodes potentially suitable for environmental potentiometric sensors | Semantic Scholar

Leakless, Bipolar Reference Electrodes: Fabrication, Performance, and Miniaturization | Analytical Chemistry
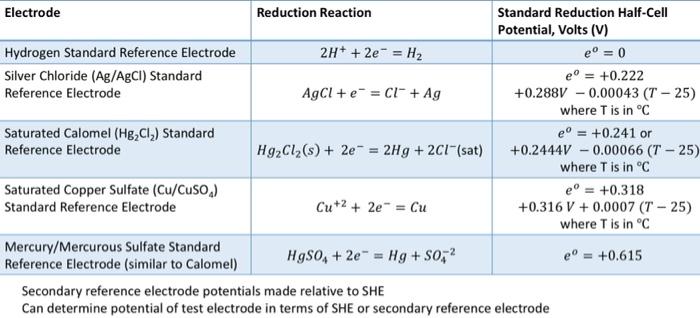
![PDF] Evaluation of Ag/AgCl-electrode standard potential uncertainty used in primary pH measurements by Monte Carlo simulation | Semantic Scholar PDF] Evaluation of Ag/AgCl-electrode standard potential uncertainty used in primary pH measurements by Monte Carlo simulation | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/dac230ae14b460452ee66bed08e8d0f57efb8f96/3-Table1-1.png)
PDF] Evaluation of Ag/AgCl-electrode standard potential uncertainty used in primary pH measurements by Monte Carlo simulation | Semantic Scholar

The standard potential of `Cl^(c-)|AgCl|Ag` half cell is related to that of `Ag^(o+)|Ag` though ... - YouTube

A screen-printed Ag/AgCl reference electrode with long-term stability for electroanalytical applications - ScienceDirect

inorganic chemistry - Where does the free electron from from in Ag/AgCl reaction? - Chemistry Stack Exchange
2 The standard half reduction potential of Ag+|Ag is 0.79V is 25^° C. Given the experimental value Ksp=1.5 10* 10 for AgCl, calculate the standard half cell reduction potential for the Ag|AgCl

The standard half cell reduction potential for Ag^+|Ag is 0.7991 V at 25^oC . Given that the experimental value of Ksp = 1.56 × 10^-10 for AgCl , calculate the standard half

The standard reduction potential of a AgCl/Ag electrode is 0.2 V and that of a silver electrode ( Ag^ + /Ag ) is 0.79 V. The maximum amount of AgCl that can