SUB04: Preparing Submissions in the Common Technical Document (CTD) Format | Zenosis – Learning for Life

ClinProve - CTD Structure The Common Technical Document is divided into five modules: 1. Administrative and prescribing information 2. Overview and summary of modules 3 to 5 3. Quality (pharmaceutical documentation) 4.

Las Conferencias Internacionales de Armonización y el Common Technical Document (CTD) | Semantic Scholar
Potential to Simplify the Writing of Submission Documents: Evaluation of Publicly Available Module 2 Documents in Drug Submissio

Text Background png download - 770*768 - Free Transparent Common Technical Document png Download. - CleanPNG / KissPNG

1 COMMON TECHNICAL DOCUMENT / ORIGIN OF CTD… ICH EWG CTD WAS OFFICIALLY SIGNED OFF IN NOVEMBER 2000, AT 5 TH ICH CONFERENCE; SAN DIEGO,CALIFORNIA. - ppt download


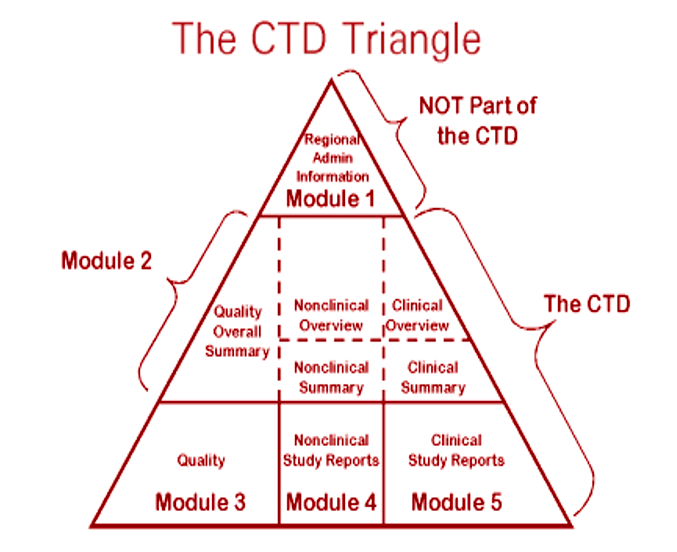










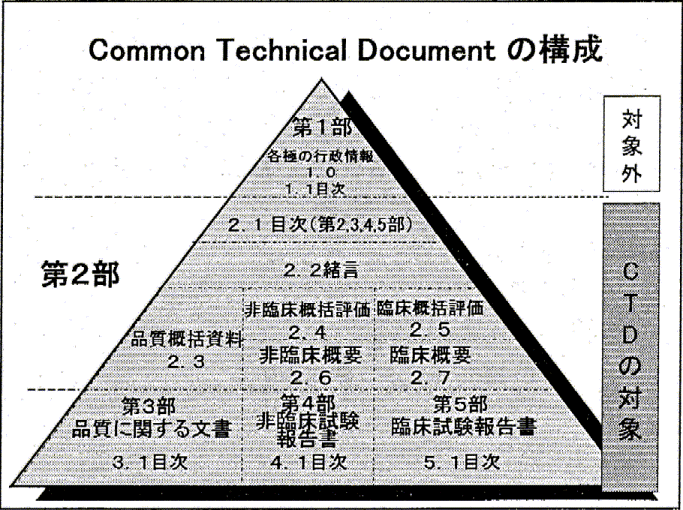
![Common technical document triangle [7] | Download Scientific Diagram Common technical document triangle [7] | Download Scientific Diagram](https://www.researchgate.net/publication/320372658/figure/fig1/AS:550839028203520@1508341666076/Common-technical-document-triangle-7.png)