Predicting clinical decline and conversion to Alzheimer's disease or dementia using novel Elecsys Aβ(1–42), pTau and tTau CSF immunoassays | Scientific Reports

An accurate fully automated panel of plasma biomarkers for Alzheimer's disease - Palmqvist - Alzheimer's & Dementia - Wiley Online Library

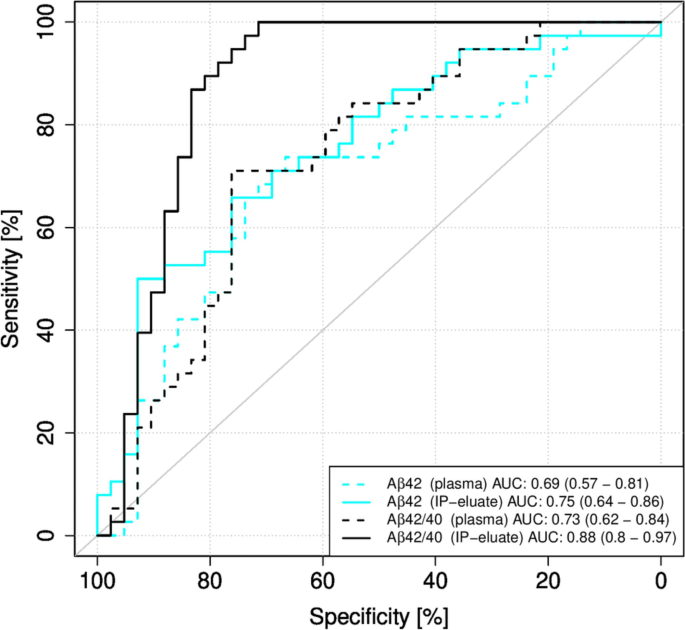
Method comparison study of the Elecsys® β-Amyloid (1–42) CSF assay versus comparator assays and LC-MS/MS - ScienceDirect

Roche bags USFDA Breakthrough Device Designation for Elecsys Amyloid Plasma Panel for early detection of Alzheimer's disease

FDA Grants Breakthrough Device Designation for Roche Alzheimer's Early-Detection Solution | Clinical Lab Products

Roche Alzheimer's disease Cerebrospinal Fluid (CSF) assays receive FDA clearance, supporting more accurate and timely diagnosis
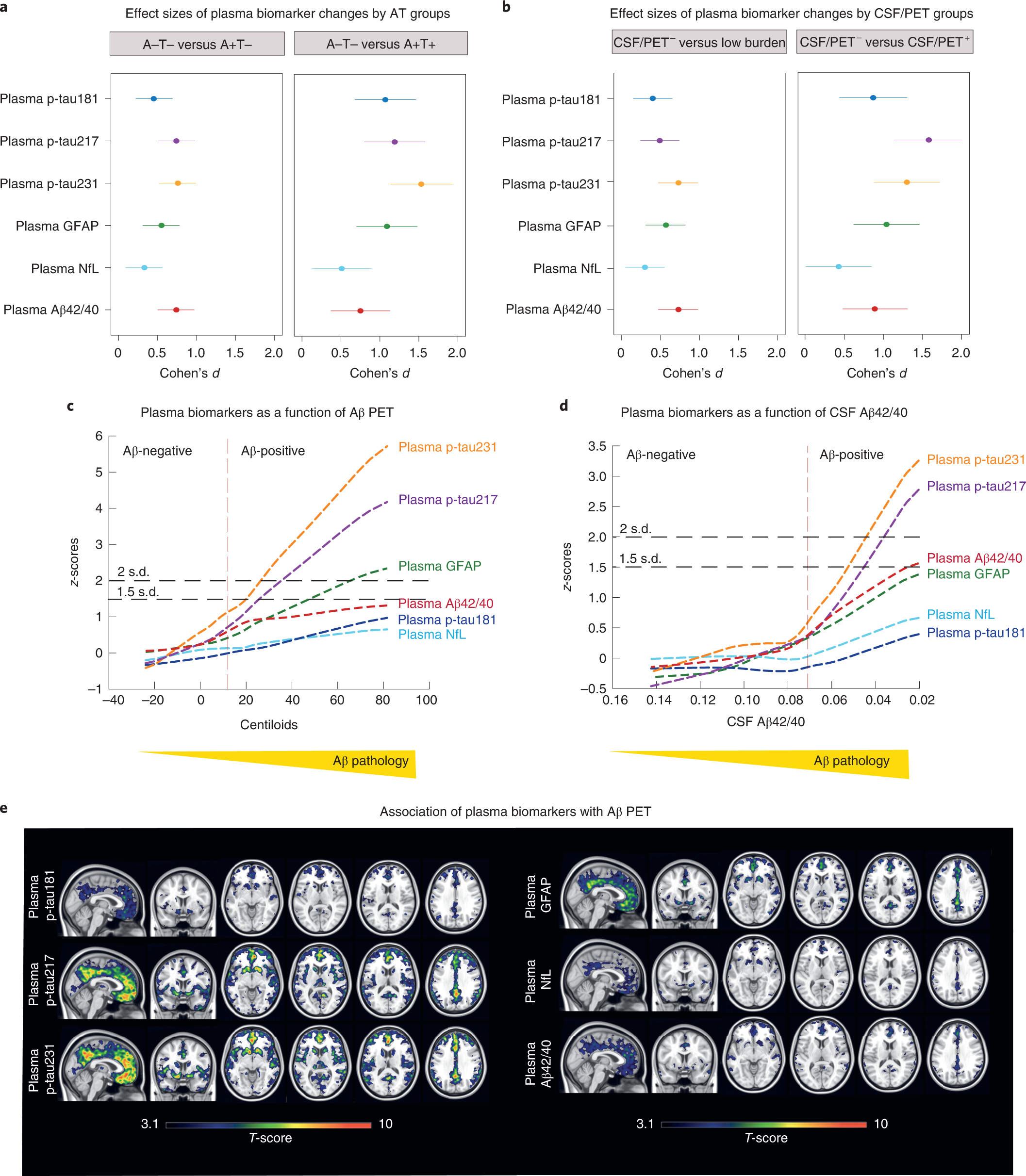
Plasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer's disease | Nature Medicine

Roche colabora con Lilly en la mejora del diagnóstico precoz de la enfermedad de Alzheimer | NGD - Noticias y Gestión de la Dependencia

Roche bags USFDA Breakthrough Device Designation for Elecsys Amyloid Plasma Panel for early detection of Alzheimer's disease

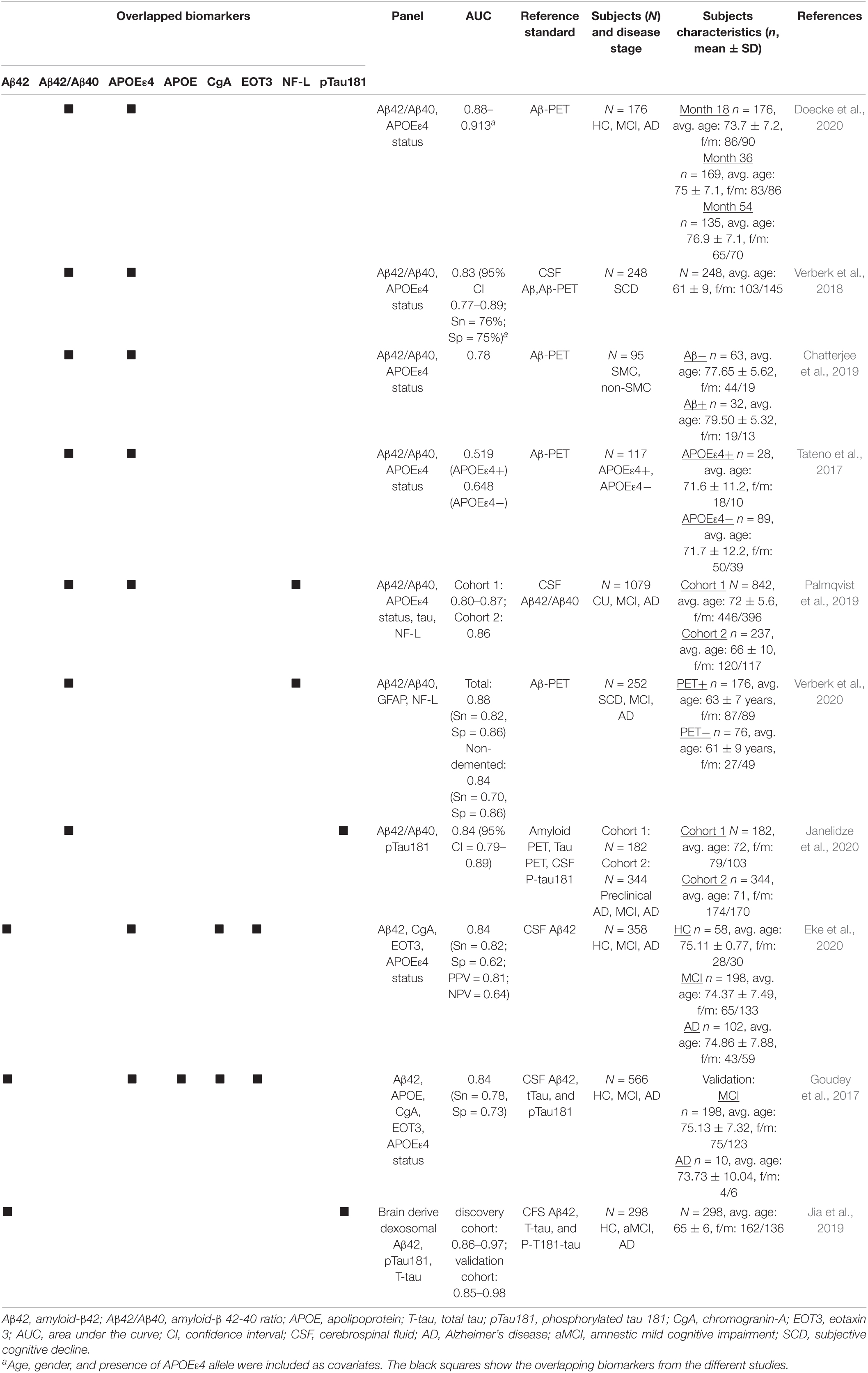
PDF) Performance of Fully Automated Plasma Assays as Screening Tests for Alzheimer Disease-Related β-Amyloid Status

Confounding factors of Alzheimer's disease plasma biomarkers and their impact on clinical performance | medRxiv

Roche's Elecsys Amyloid Plasma Panel Granted FDA Breakthrough Device Designation to Enable a Timely Diagnosis of Alzheimer's Disease