The freezing point of urea solution is -0.60C. How much urea is required to be dissolved in 3kg of water (Of = 1.50C kg/ mol)? - Quora

What is the freezing point of a solution that contains 20.2 g of urea CO(NH_2)_2 in 295 mL water? (Assume a density of water of 1.00 g/mL) | Homework.Study.com

An aqueous solution of urea has freezing point of -0.604^(@)C & At 27^(@)C . The osmatic pressure of the same solution is atm . (assume molality and molarity are same)

Welcome to Chem Zipper.com......: An aqueous solution has 5% urea and 10% glucose by weight. what will be the freezing point of this solution ? ( Kf = 1.86 K Kg per mole)

Urea, (NH_2)_2CO, is dissolved in 73.5 g of water. The solution freezes -0.095^0C. How many grams of urea were dissolved to make this solution? (Answer must be in grams) | Homework.Study.com
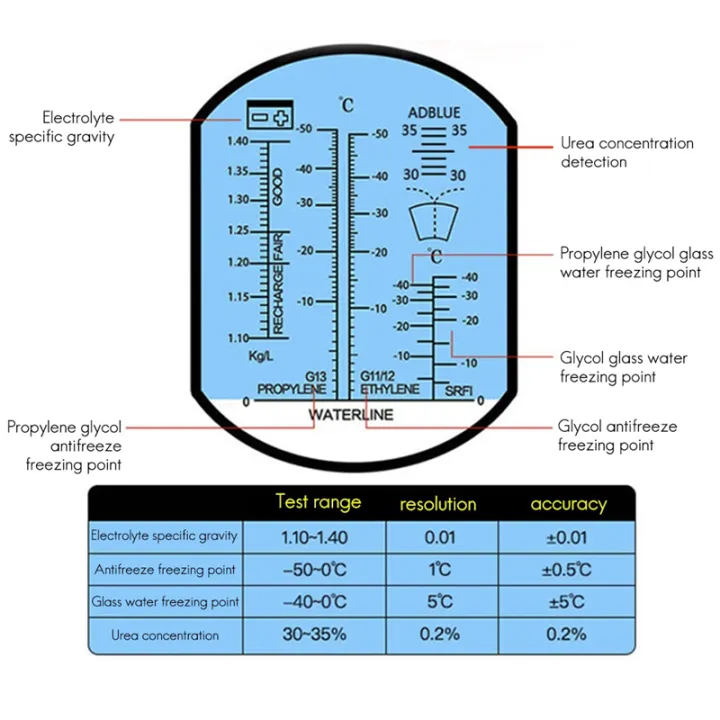
1 Piece Detector Freezing Point Detector for Vehicle Urea Freezing Point Detector: Buy Online at Best Prices in Pakistan | Daraz.pk

AABBCCDDEEMSMSMSMSMS 2006 2.8 1.3 3.3 2.2 2.6 1.7 3.0 2.2 1.9 1.4 2007 3.2 2.0 2.4 1.6 2.2 1.5 2.5 1.9 2.0 1.7 2008 1.9 0.9 2.9 1.6 2.1 1.0 2.3 1.5 1.6 1.1 2009 1.0 0.4 2.4 1.3 2.8 1.4 2.1 1.2 3.2 2.5 2010 2.5 1.5 2.3 1.2 2.6 2.1 1.8 1.1 3.1 2.6 What ...

SOLVED: certain substance [ has normal freezing point of 6.1 C and molal freezing point depression constant K5 =4,69 'C.kg mol point of solution made of 15. g of urea ((NH,), co)

The freezing point of aqueous solution that contains `3%` of urea, `7.45%` KCl and `9%` of glucose - YouTube

Means and standard errors of milk urea nitrogen (MUN), freezing point... | Download Scientific Diagram

An aqueous solution of urea has a freezing point of -0 40C Calculate the osmotic pressure of the same - Chemistry - Solutions - 13527667 | Meritnation.com

An aqueous solution of urea freezes at - 0.186^o C. Kf for water = 1.86 K kg mol^-1 , Kb for water = 0.512 K kg mol^-1 . The boiling point of urea solution will be :
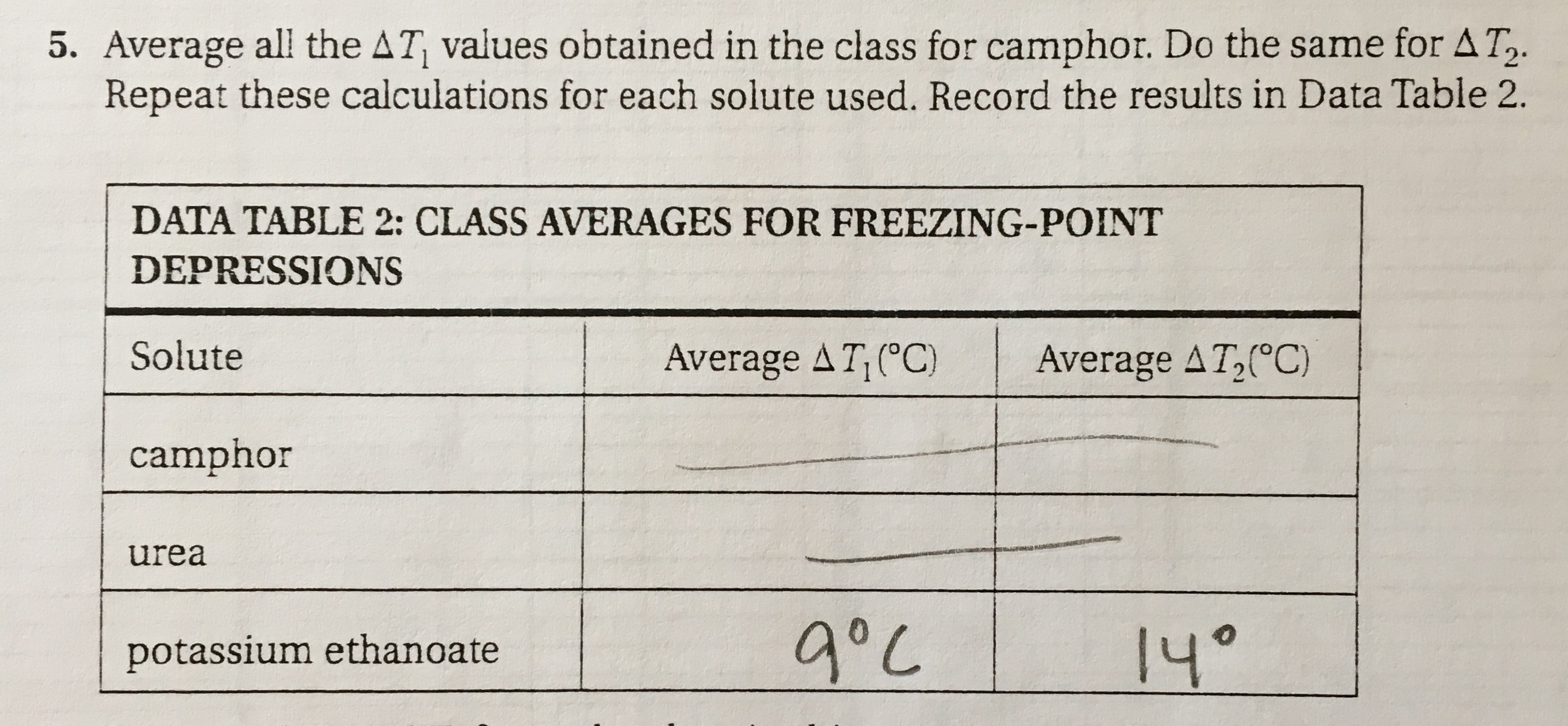
The freezing point depression of potassium ethanoate is greater than that of the other two solutes (camphor and urea) when an equal number of moles of each is used. Can somebody explain
![Freezing point of Urea solution is –0.60C. How much Urea (in g) is required to dissolve in 3 Kg of water [Kf = 1.50C Kg/mol]Correct answer is between '71,73'. Can you explain Freezing point of Urea solution is –0.60C. How much Urea (in g) is required to dissolve in 3 Kg of water [Kf = 1.50C Kg/mol]Correct answer is between '71,73'. Can you explain](https://edurev.gumlet.io/ApplicationImages/Temp/547e1bf8-9a5d-466f-808b-51055774211f_lg.jpg?w=360&dpr=2.6)
Freezing point of Urea solution is –0.60C. How much Urea (in g) is required to dissolve in 3 Kg of water [Kf = 1.50C Kg/mol]Correct answer is between '71,73'. Can you explain
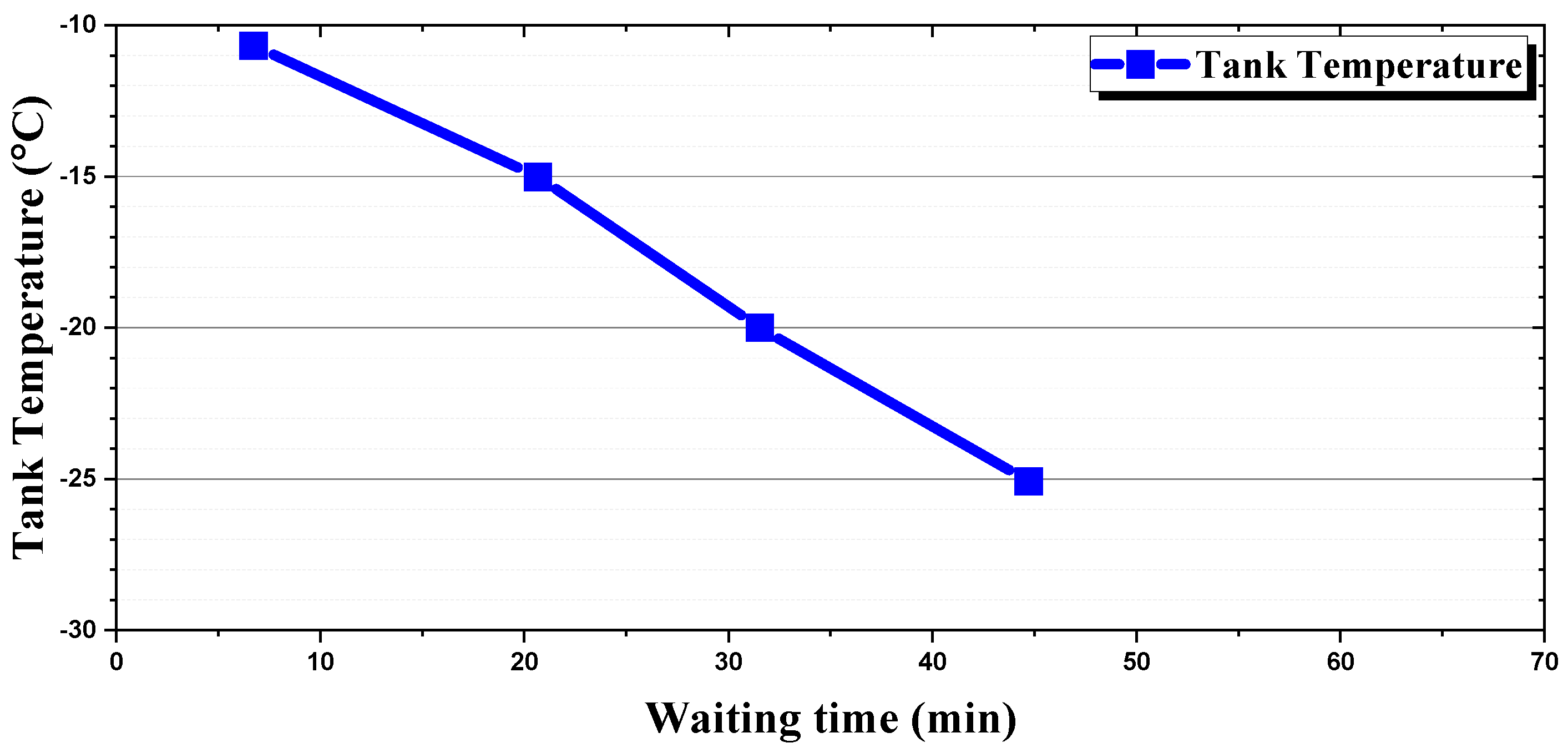
Energies | Free Full-Text | Melting and Heat Transfer Characteristics of Urea Water Solution According to a Heating Module’s Operating Conditions in a Frozen Urea Tank