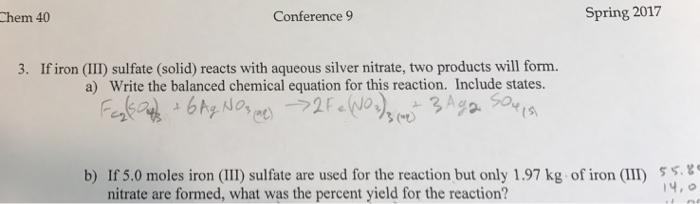
SOLVED: Silver nitrate reacts with iron (III) chloride to produce silver chloride and iron (III) nitrate. In a particular experiment, a solution containing 25.0 g of silver nitrate is completely reacted. Write

An iron nail is dipped in a silver nitrate solution. After some time, the solution turns green due to the formation of iron nitrate and silver. Which of the following equations represents

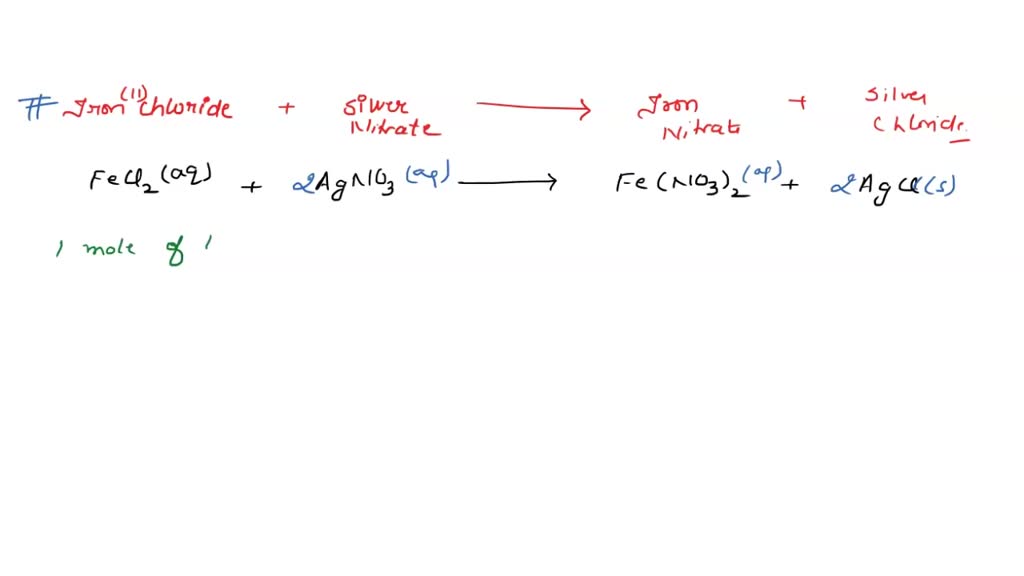
SOLVED: 41, Silver nitrate, AgNO3(aq) , reacts with iron(III) chloride, FeCl;(aq), to produce silver chloride; 'AgCl(s), and iron(III) nitrate; Fe(NOz)3(aq) 3AgNOs(aq) - FeClz(aq) 3AgCl(s) + Fe(NOs)s(aq) a. Ifa solution containing 18.00 g

Fe+AgNO3=Fe(NO3)2+Ag Balanced Equation||Iron+Silver nitrate=Iron(ii)nitrate+ Silver Balanced Equation - YouTube
How to write a balanced chemical equation for the reaction between silver nitrate and aluminum? What type of reaction is this - Quora

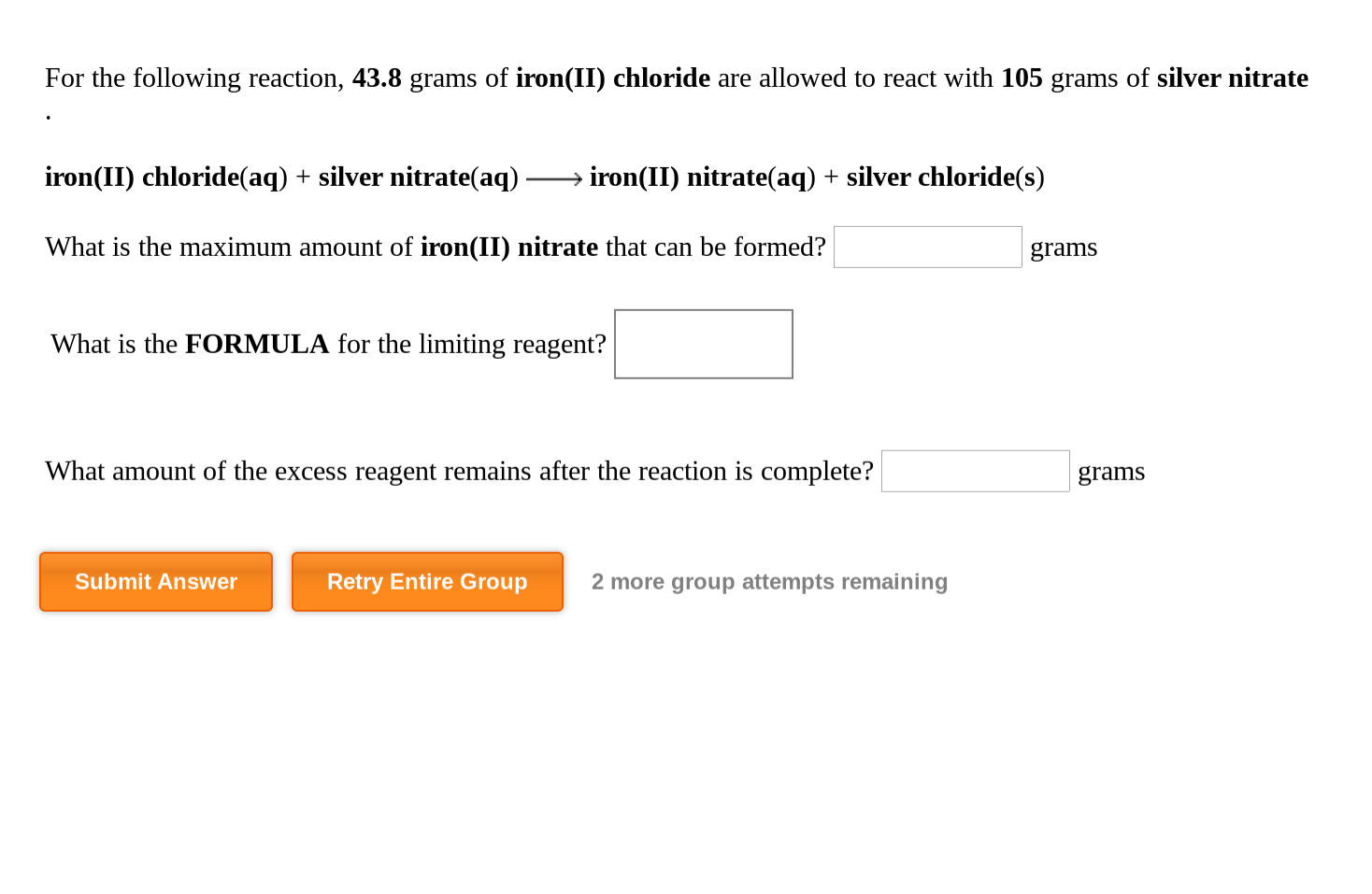
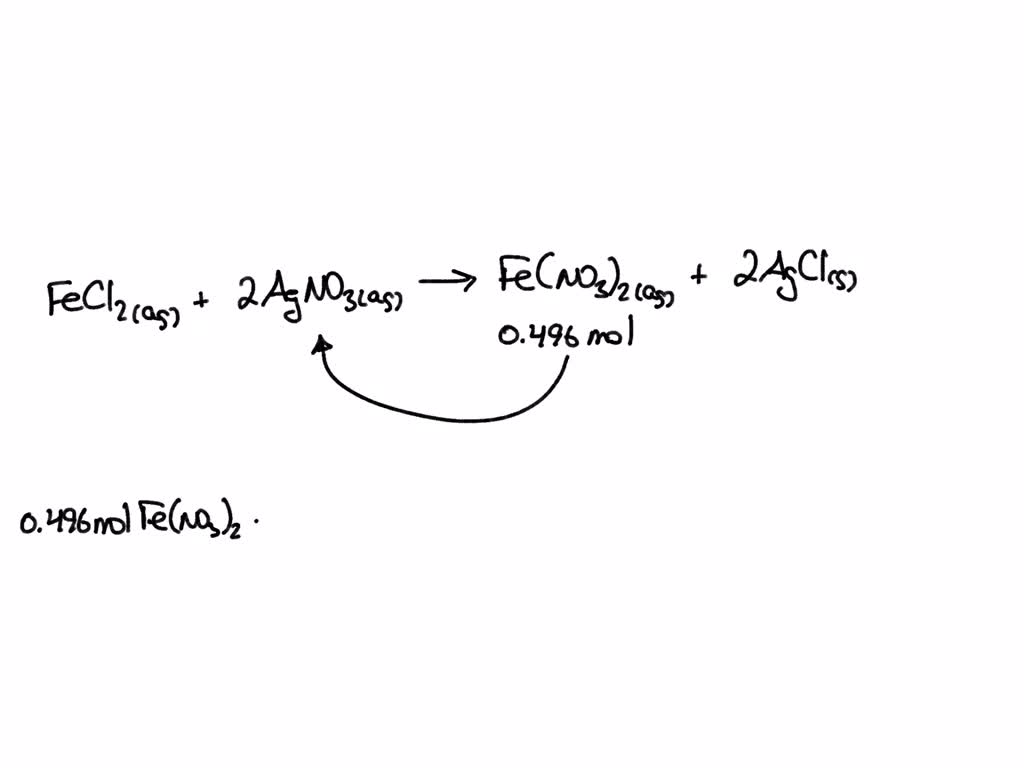
SOLVED: Silver nitrate, AgNO3 reacts with iron(III) chloride, FeCl3 to give silver chloride AgCl, and iron(III) nitrate, Fe(NO3)3- solution containing 18.1 of AgNO3 was mixed with solution containing 30.6 of FeCl3. How