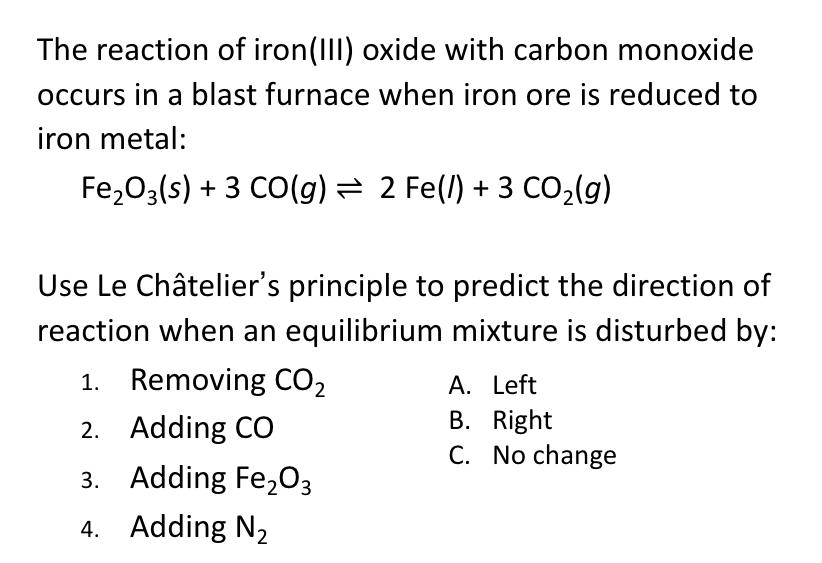
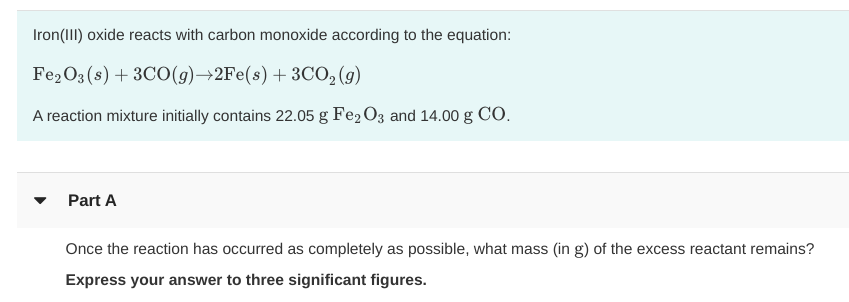
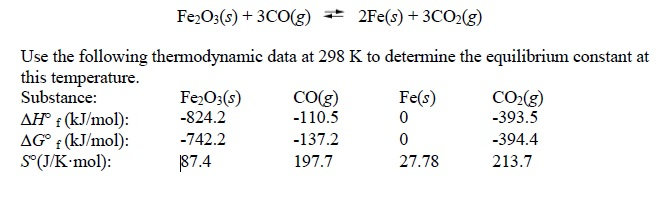
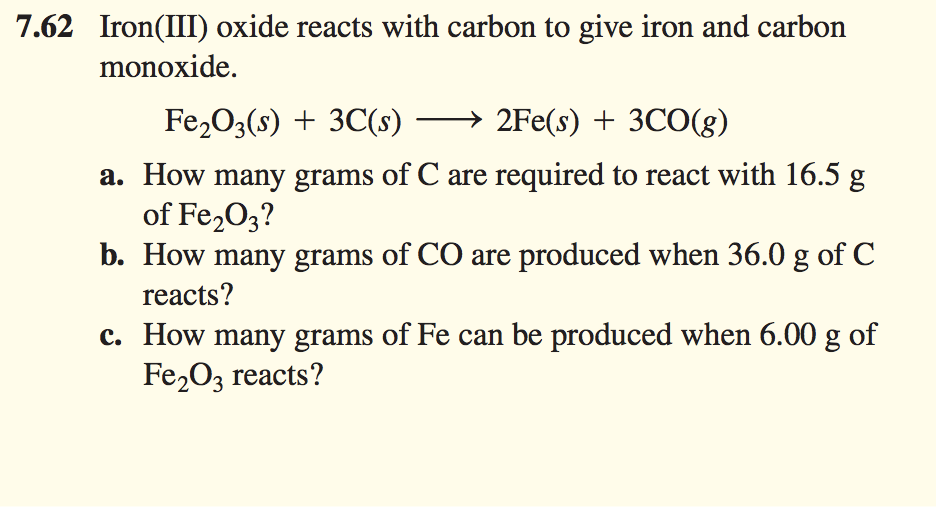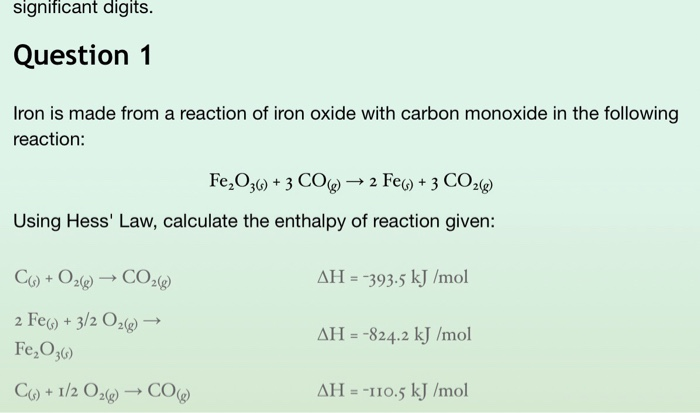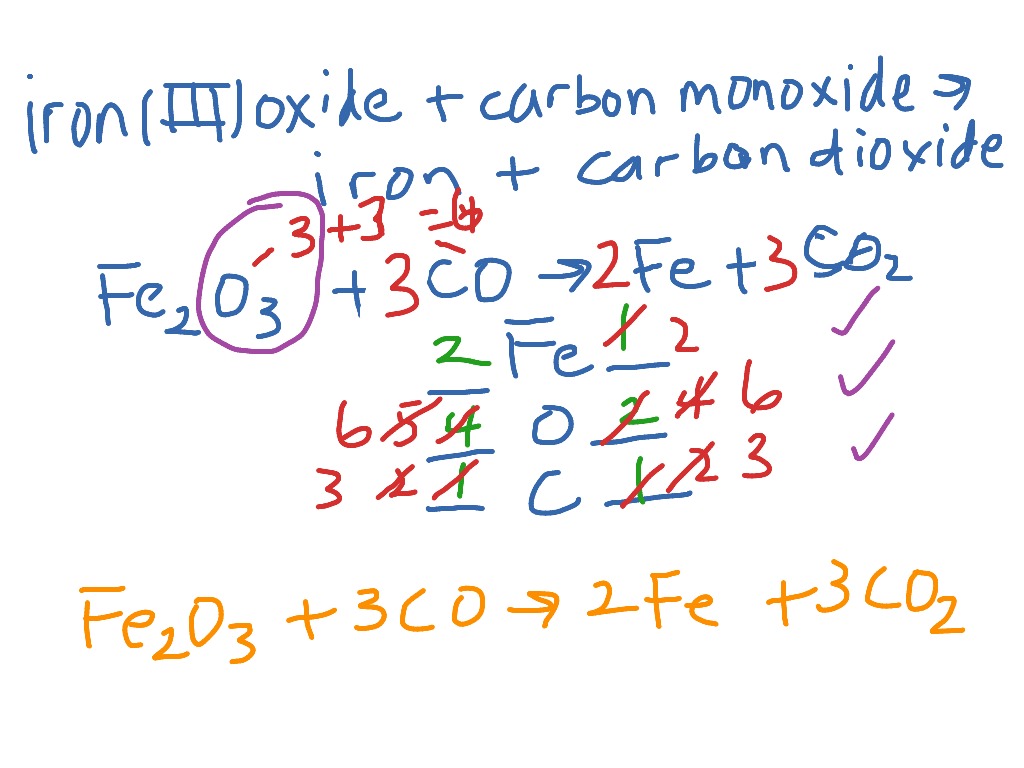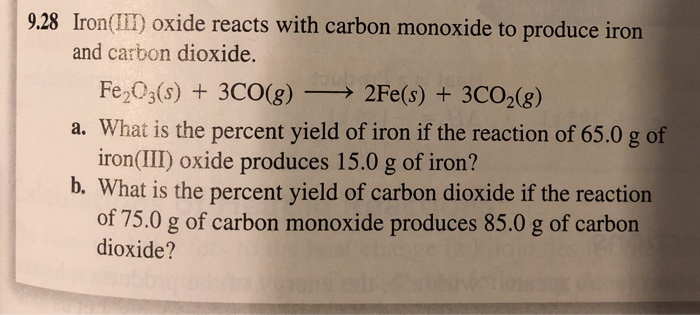SOLVED: Question 1 5 pts Iron (III) oxide reacts with carbon monoxide to produce iron metal and carbon dioxide: FezOz + 3COD2Fe + 3C02 Ifa student starts with 1.820 grams of carbon

Tuesday January 25, 2011 (Types of Chemical Reactions; Predicting the Products of Chemical Reactions) - ppt download
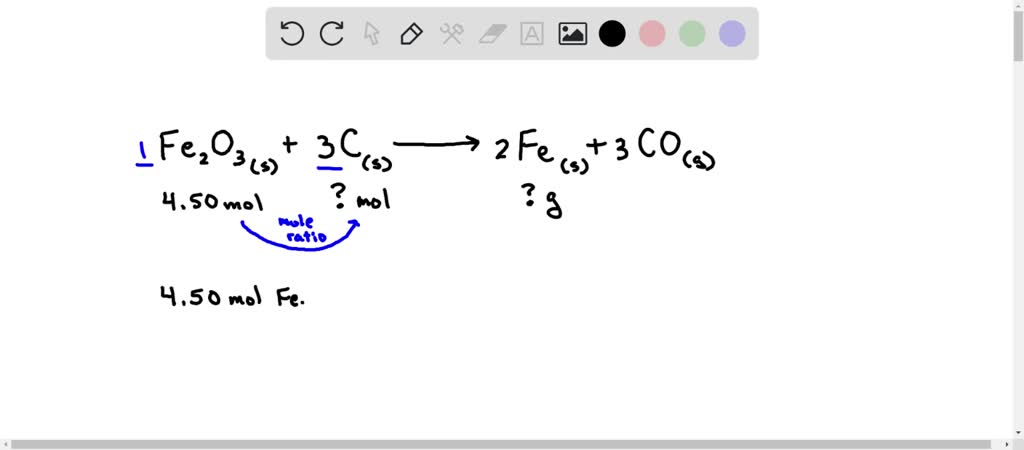
SOLVED: Solid iron(III) oxide reacts with carbon to produce solid iron and carbon monoxide gas. If 4.50 moles iron(III) oxide react, how many moles of carbon are needed to react in stoichiometric

Iron(iii)oxide+Carbon monoxide=Iron+ Carbon dioxide Balanced Equation||Fe2O3+CO=Fe+CO2 Balanced Equ. - YouTube

SOLVED: Pure molten Iron and carbon monoxide are produced in a blast furnace by the reaction of iron(iii) oxcide and coke (pure elementa carbon) If 2.21 kg of pure iron(iii) oxide is
28. Magnetite is an iron oxide ore, which reacts with carbon monoxide to give iron metal and carbon dioxide. When a sample of magnetite is allowed to react with sufficient carbon monoxide,

Iron is extracted from iron oxide using carbon monoxide as shown.iron oxide + carbon monoxide → iron + carbon dioxideWhich statement is correct?
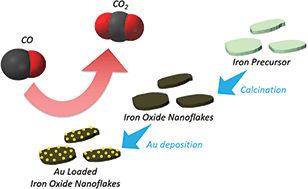
Room temperature carbon monoxide oxidation based on two-dimensional gold-loaded mesoporous iron oxide nanoflakes - Chemical Communications (RSC Publishing)

PPT - #29 When 84.8 g of iron (III) oxide reacts with excess of carbon monoxide, iron is produced. PowerPoint Presentation - ID:4448728
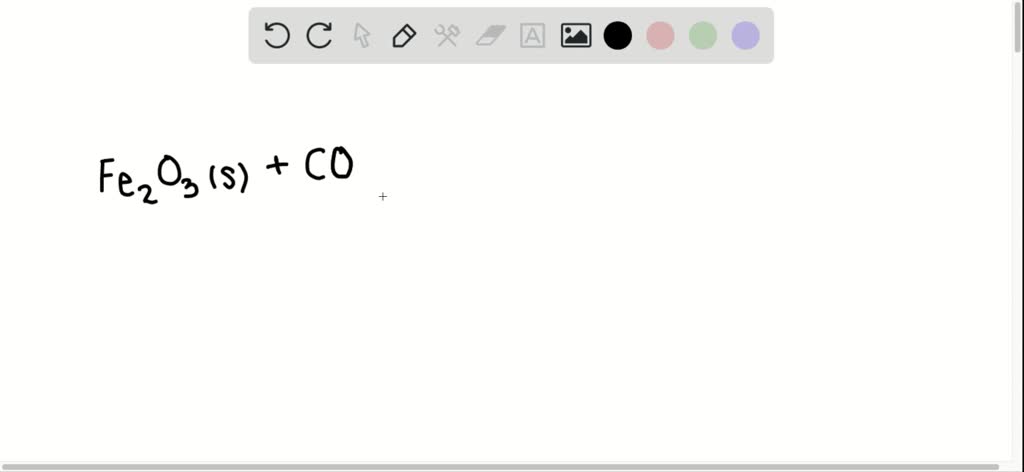
SOLVED:If ferric oxide is heated strongly in a stream of carbon monoxide gas, it produces elemental iron and carbon dioxide gas. Write the unbalanced chemical equation for this process.