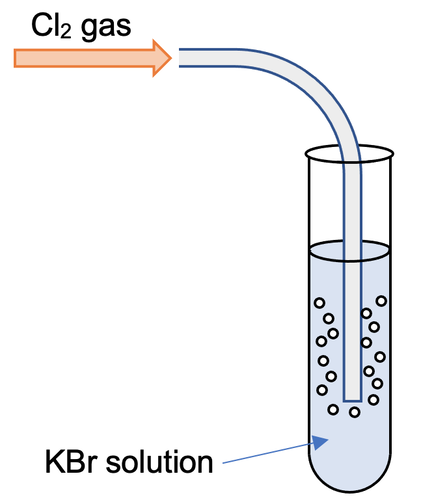
What is the balanced chemical equation for this reaction: Gaseous chlorine reacts with an aqueous solution of potassium bromide to form liquid bromine and an aqueous solution of potassium chloride? | Socratic
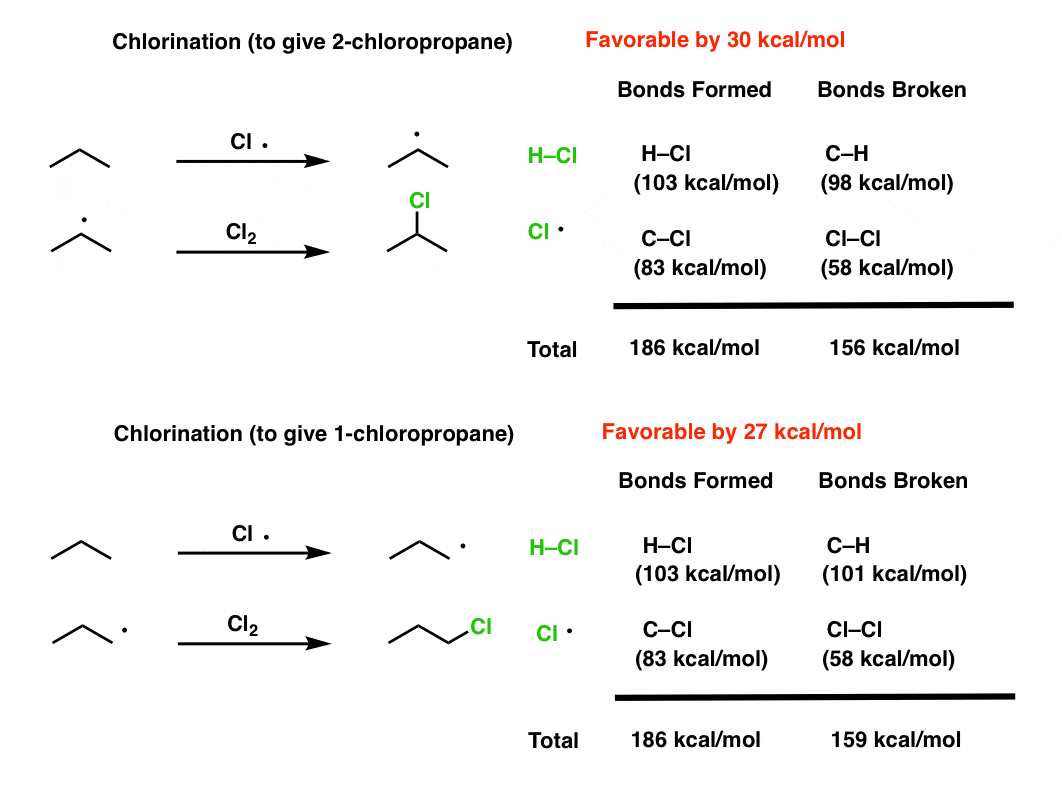
Why is sulfur less reactive than chlorine even though it is more electronegative than fluorine and bromine (which are very reactive)? - Quora

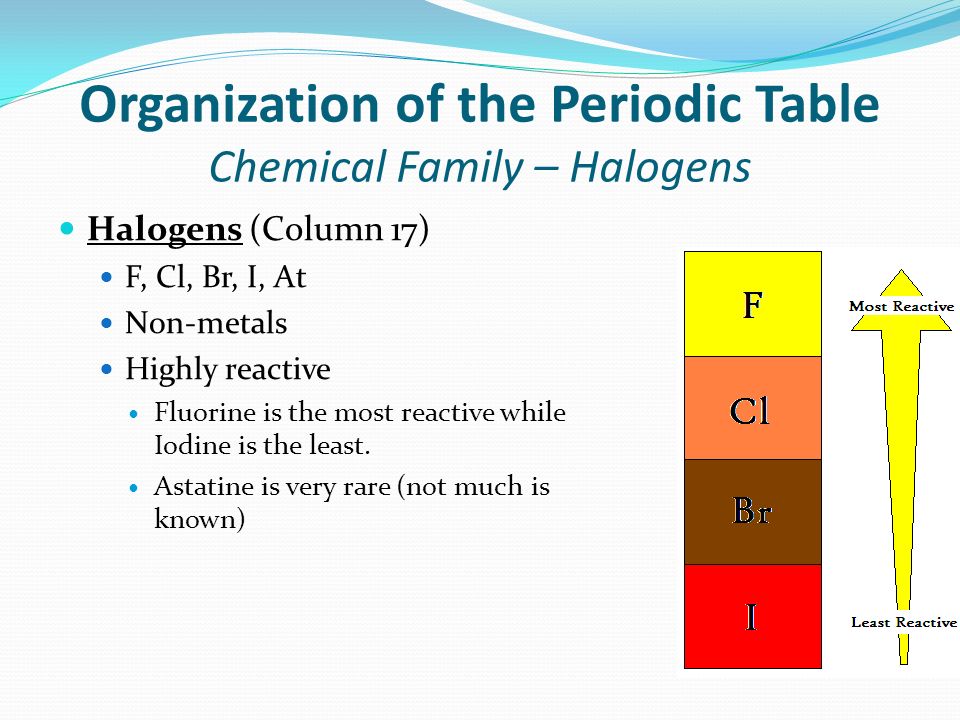
Group 7 HALOGENS fluorine chlorine bromine iodine physical properties balanced equations chemical reactions balanced gcse chemistry revision notes KS4 science igcse O level

Question Video: Showing the Color Change That Occurs When a Bromide Solution Is Converted to Bromine Gas in a Halogen Displacement Reaction | Nagwa

Fluorine is more reactive than chlorine because | 12 | THE HALOGEN FAMILY | CHEMISTRY | DINESH P... - YouTube

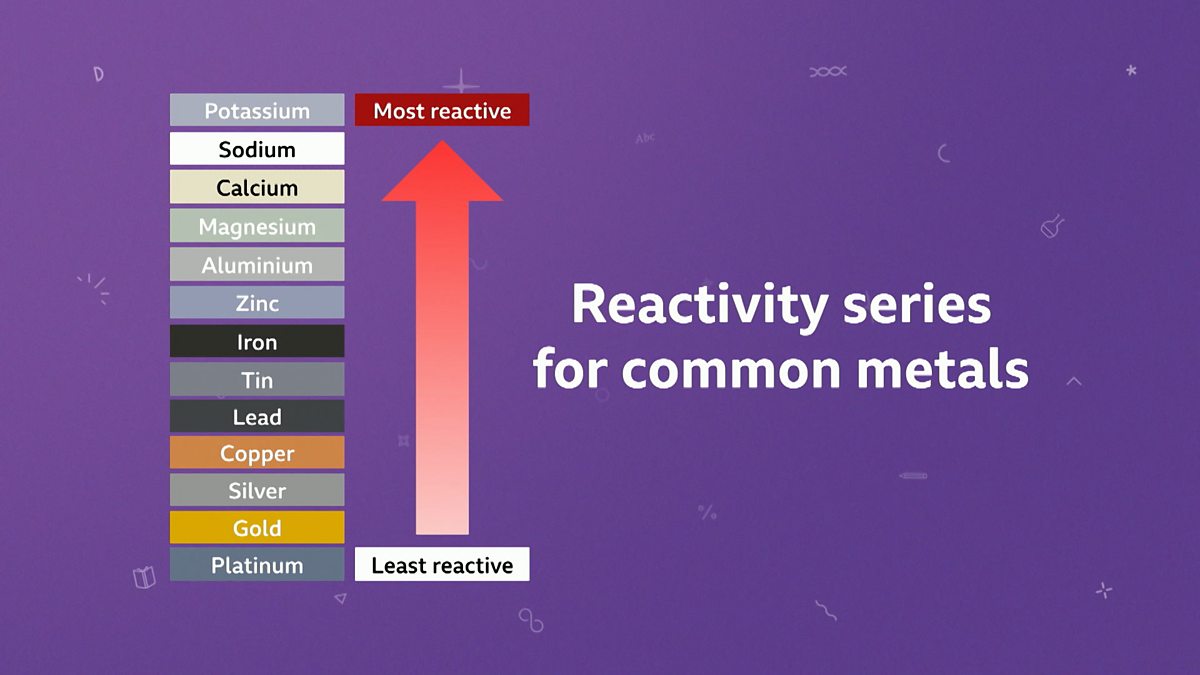
Single displacement reactions only occur when the displaced element is less reactive than the one replacing it. We can predict if a reaction will. - ppt download



![Chlorine is more reactive than [fluorine/iodine] Chlorine is more reactive than [fluorine/iodine]](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/643924934_web.png)









![What is Displacement reaction| Halogens [Online Video] – O Level Secondary Chemistry Tuition What is Displacement reaction| Halogens [Online Video] – O Level Secondary Chemistry Tuition](https://i.ytimg.com/vi/gVRX2LlCsAs/maxresdefault.jpg)