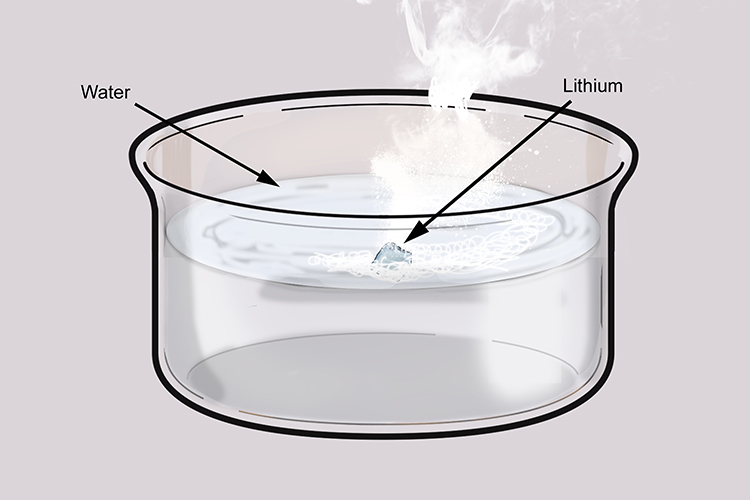
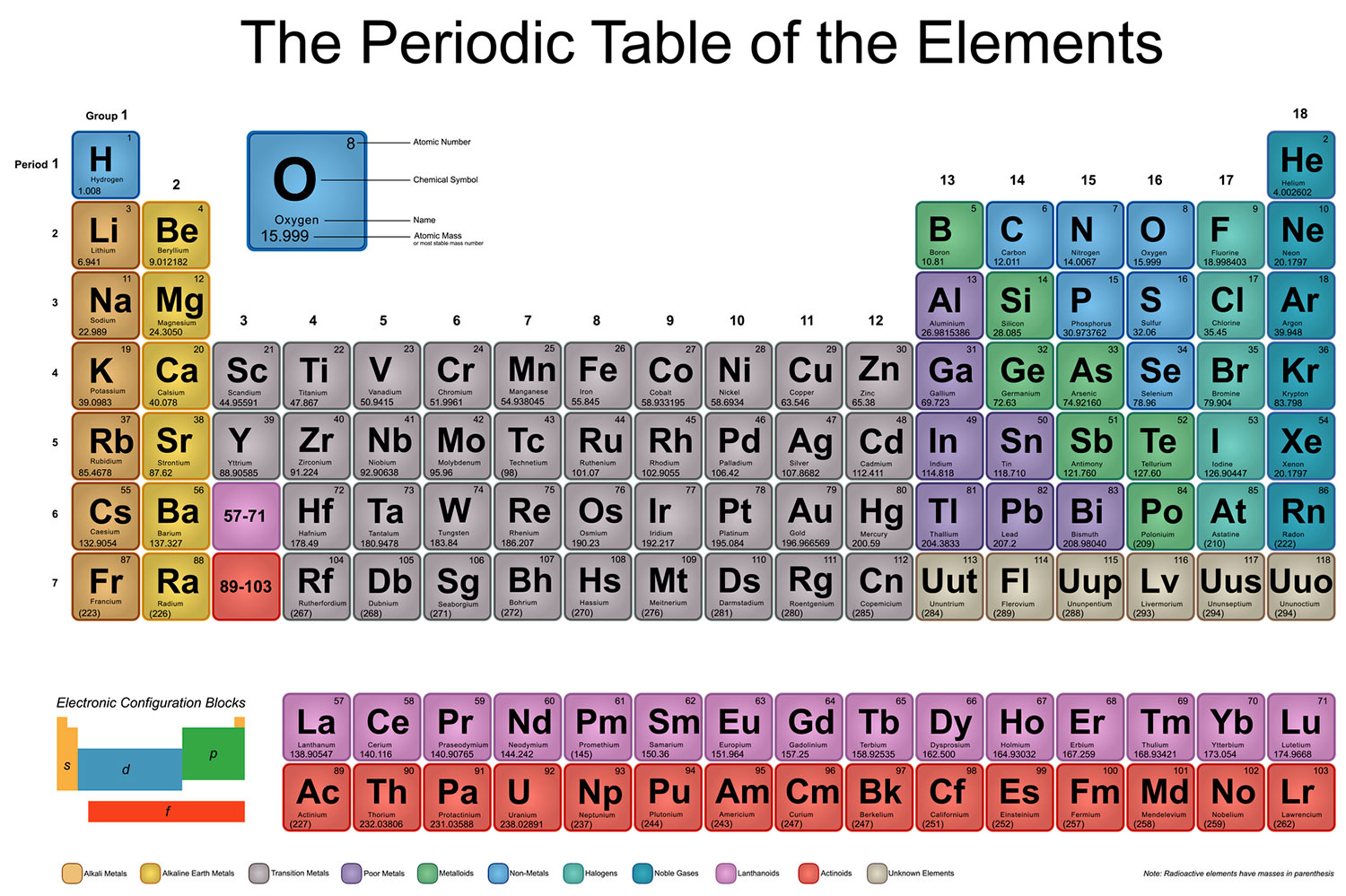
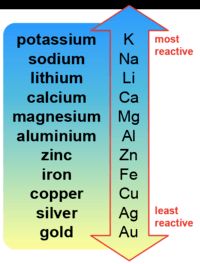
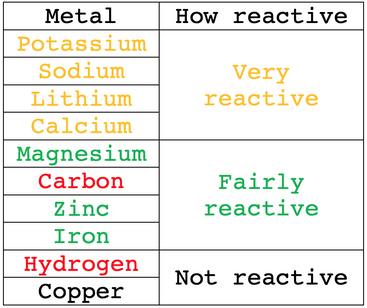
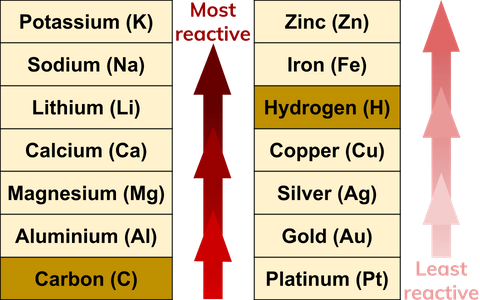
Lithium reacting with water. Lithium (Li) available as Framed Prints, Photos, Wall Art and Photo Gifts #6294599
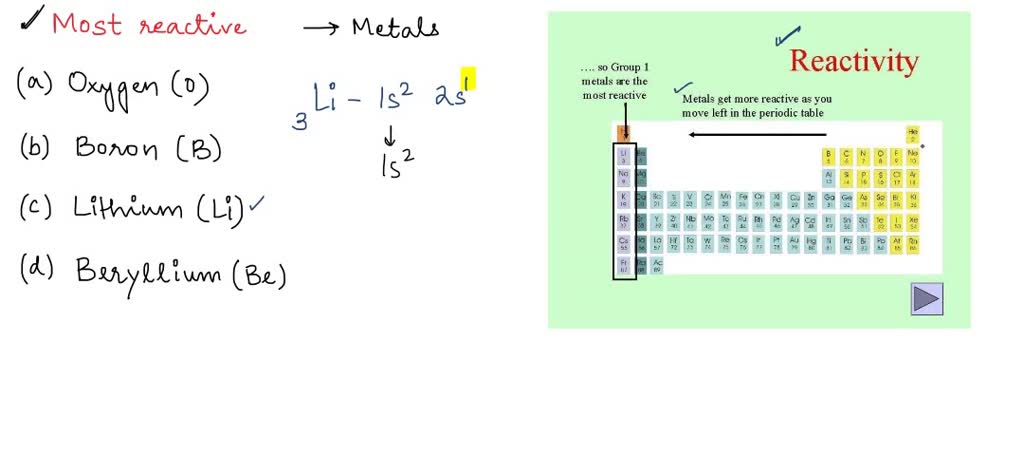
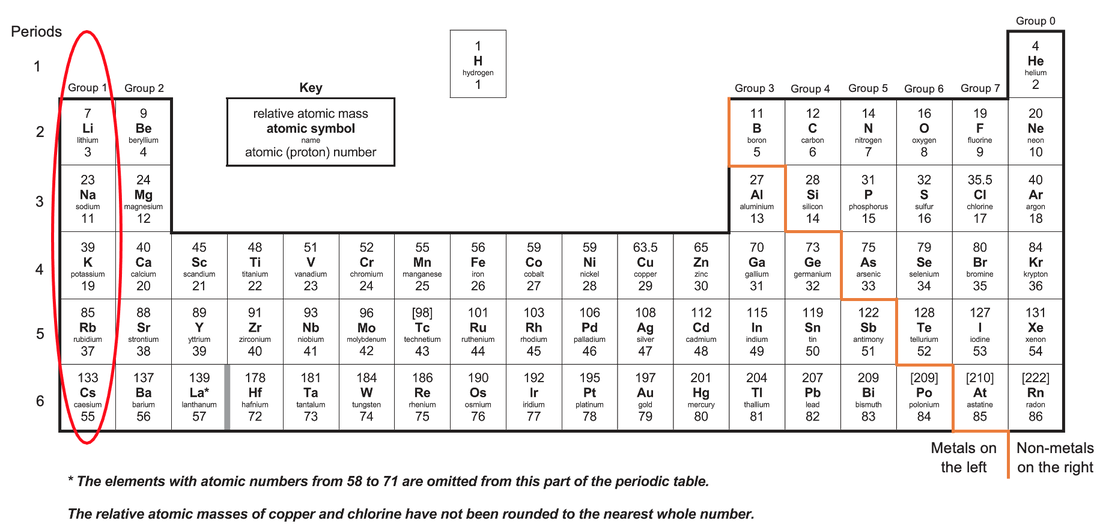
SOLVED: 'Which of the following elements would you expect to be most reactive, based on the number of valence electrons it has? A Oxygen (0) B. Boron (B) C Lithium (Li) D.

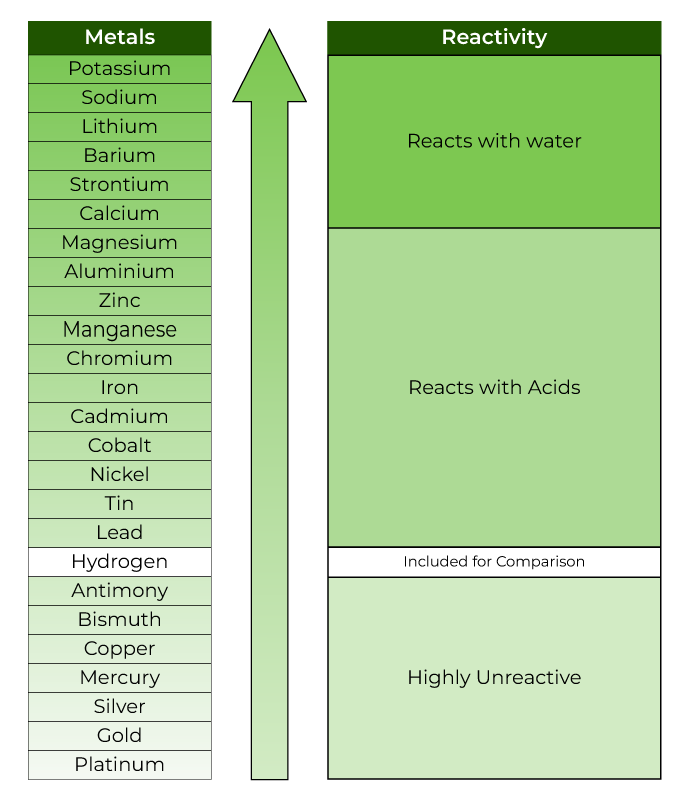
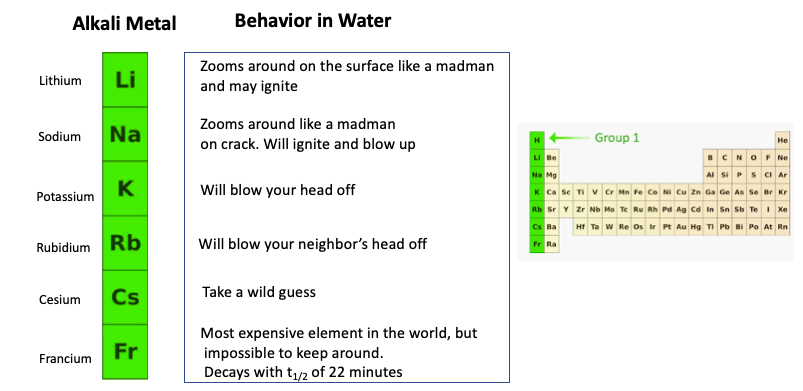
In group 1 (alkali metals), sodium is more reactive than lithium, or lithium is less reactive than sodium. Give reason.
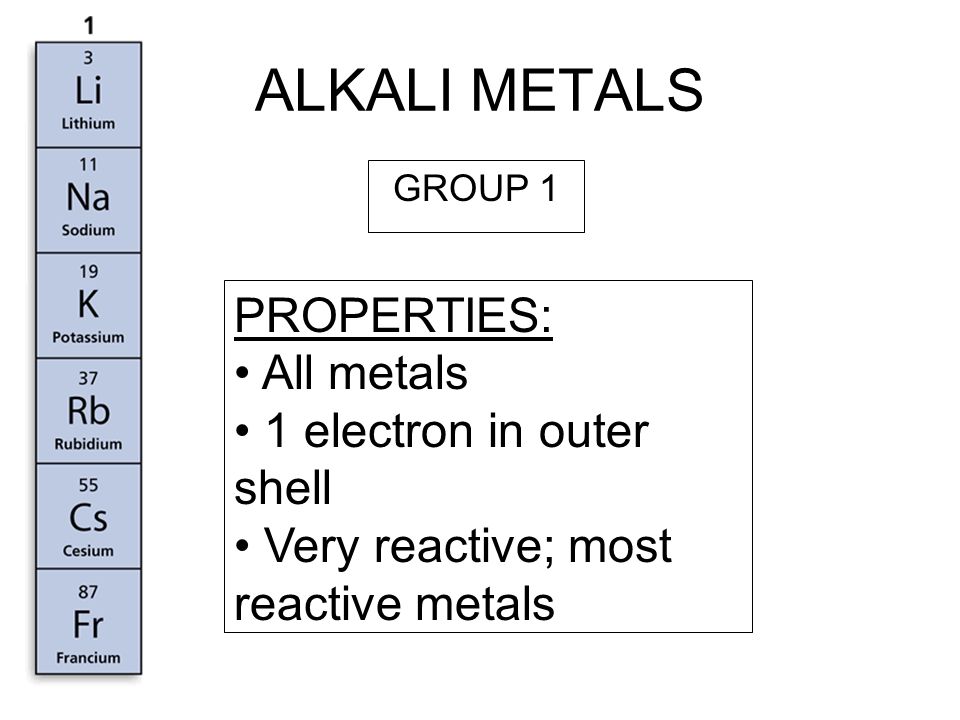
Ares Strategic Mining Inc. on Twitter: "#DidYouKnow Lithium is the least reactive of the alkali metals? https://t.co/xrNaong3ub" / Twitter