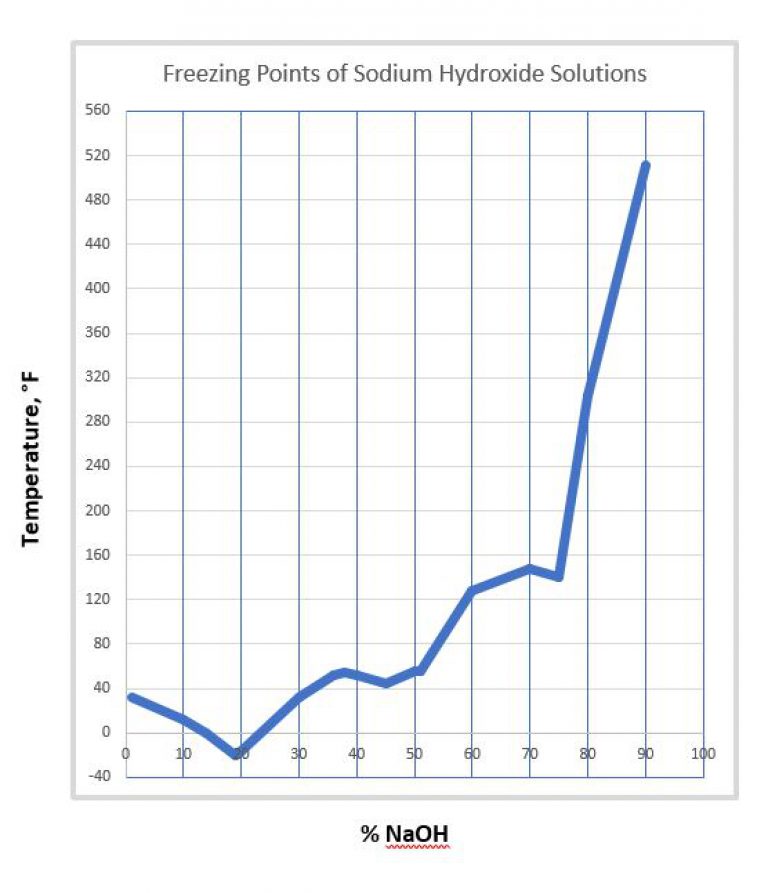
Fig. C-1. Boiling and solidifying temperatures of aqueous caustic soda... | Download Scientific Diagram

1.0 g of a monobasic acid HA in 100 g water lowers the freezing point by 0.155 K. IF 0.75 g, of same acid requires 25 mL of N/5 NaOH solution for

Arrange the following in increasing order of freezing point- 0.2 M NaOH, 0.2M Na2CO3 , 0.1M AgNO3 , 0.1M - Brainly.in

Structure and properties of nanocrystalline nickel prepared by selective leaching at different temperatures

Estimation of Freezing Point Depression, Boiling Point Elevation, and Vaporization Enthalpies of Electrolyte Solutions | Industrial & Engineering Chemistry Research