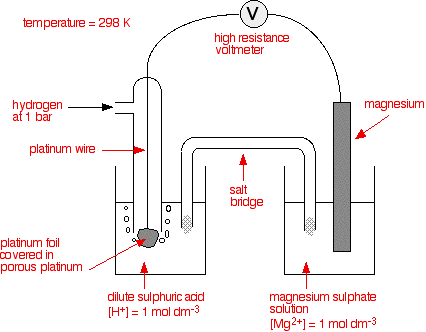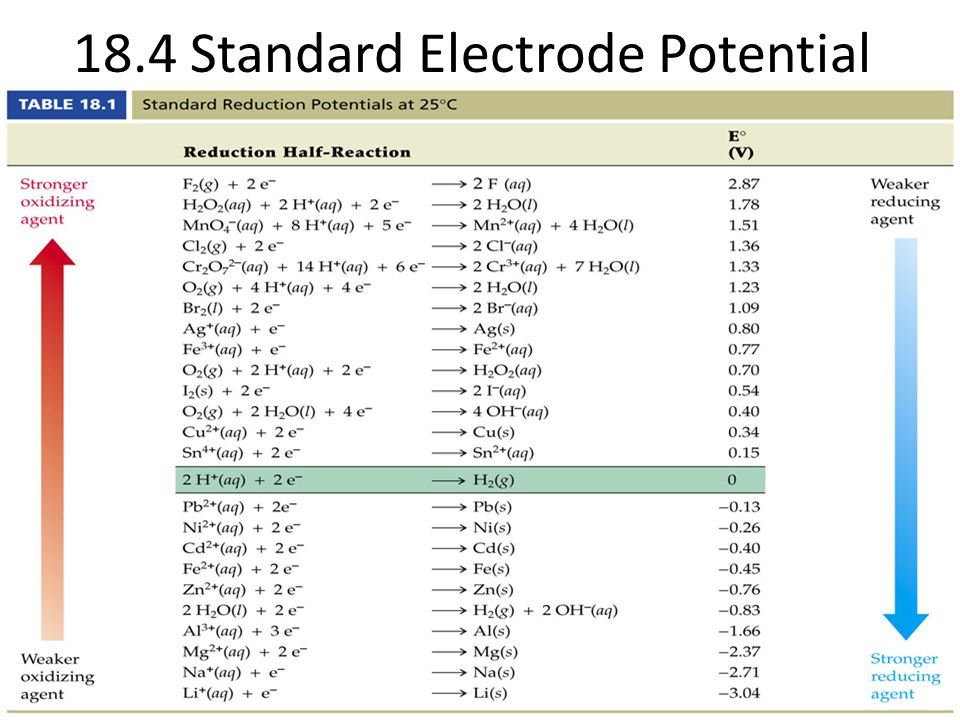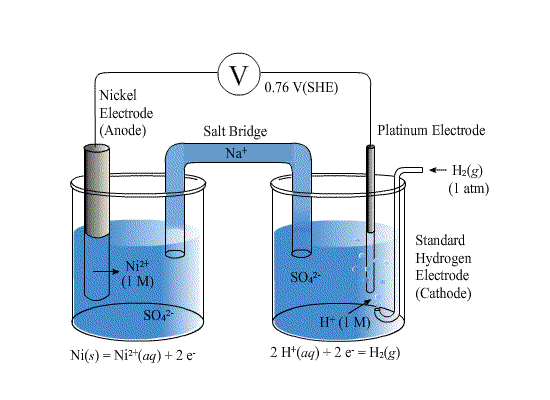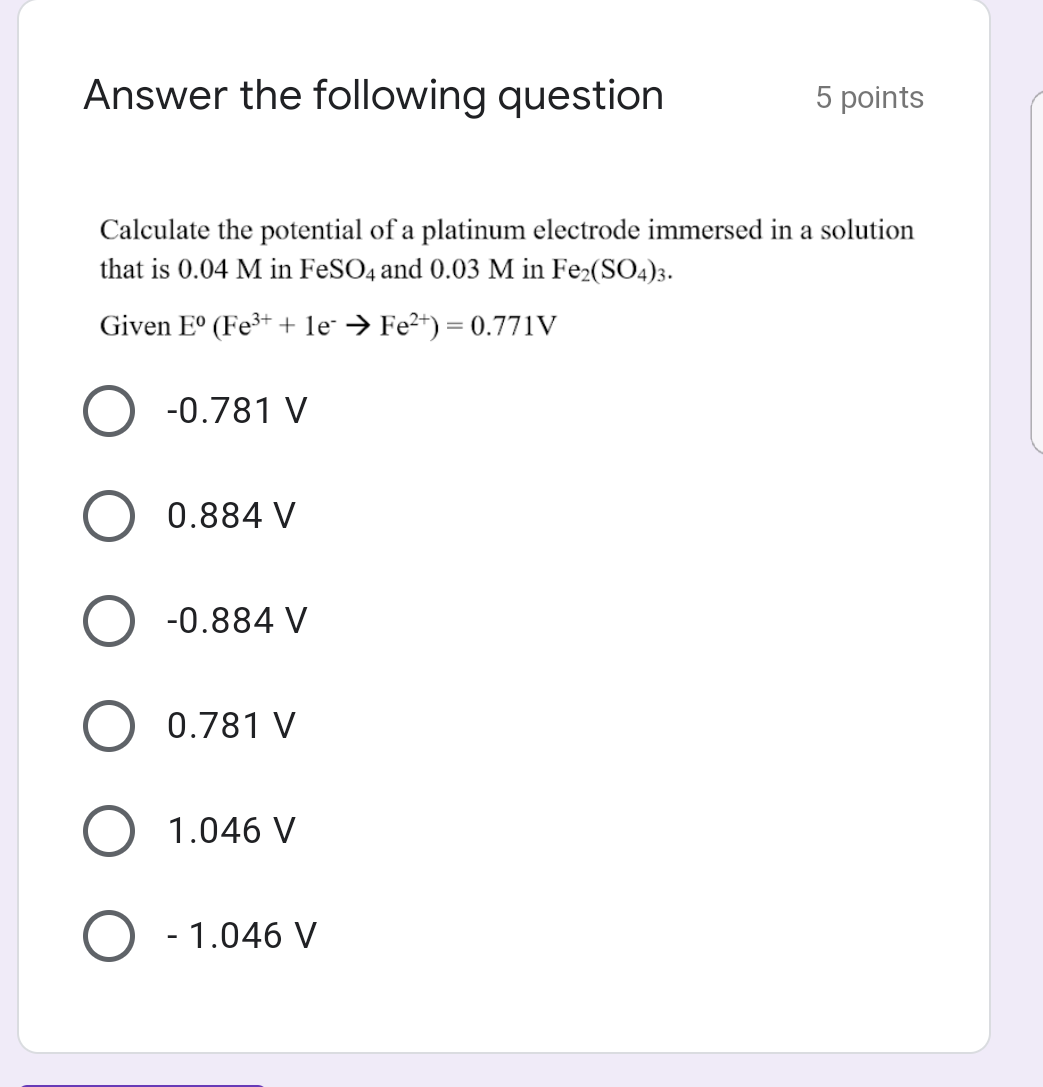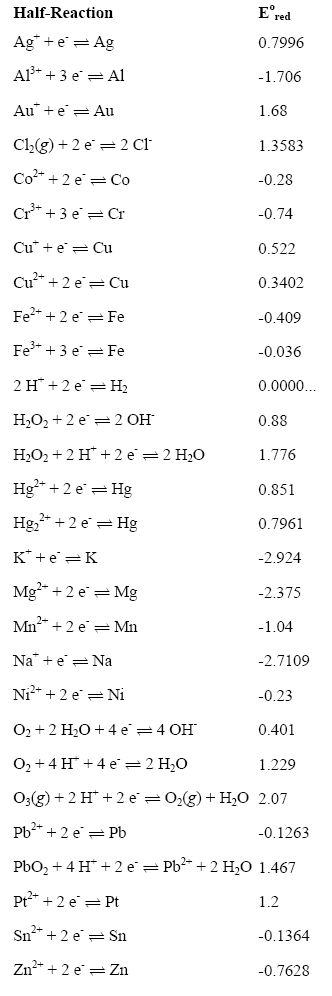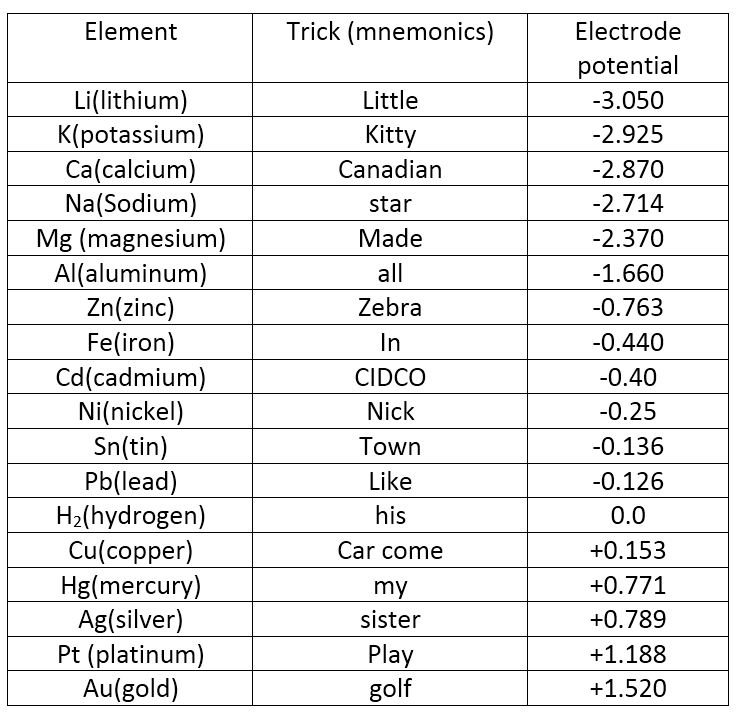
what trick one must use to remember whole emf series with its standard reduction potential values n62qc0rr -Chemistry - TopperLearning.com

A platinum electrode is immersed in a solution containing 0.1 M Fe^+2 and 0.1 M Fe^+3 . It is coupled with SHE. Concentration of Fe^+3 is increased to 1.0 M without change

Change in potential of a mechanically polished platinum electrode with... | Download Scientific Diagram

Applicability of Platinum as a Counter-Electrode Material in Electrocatalysis Research | ACS Catalysis

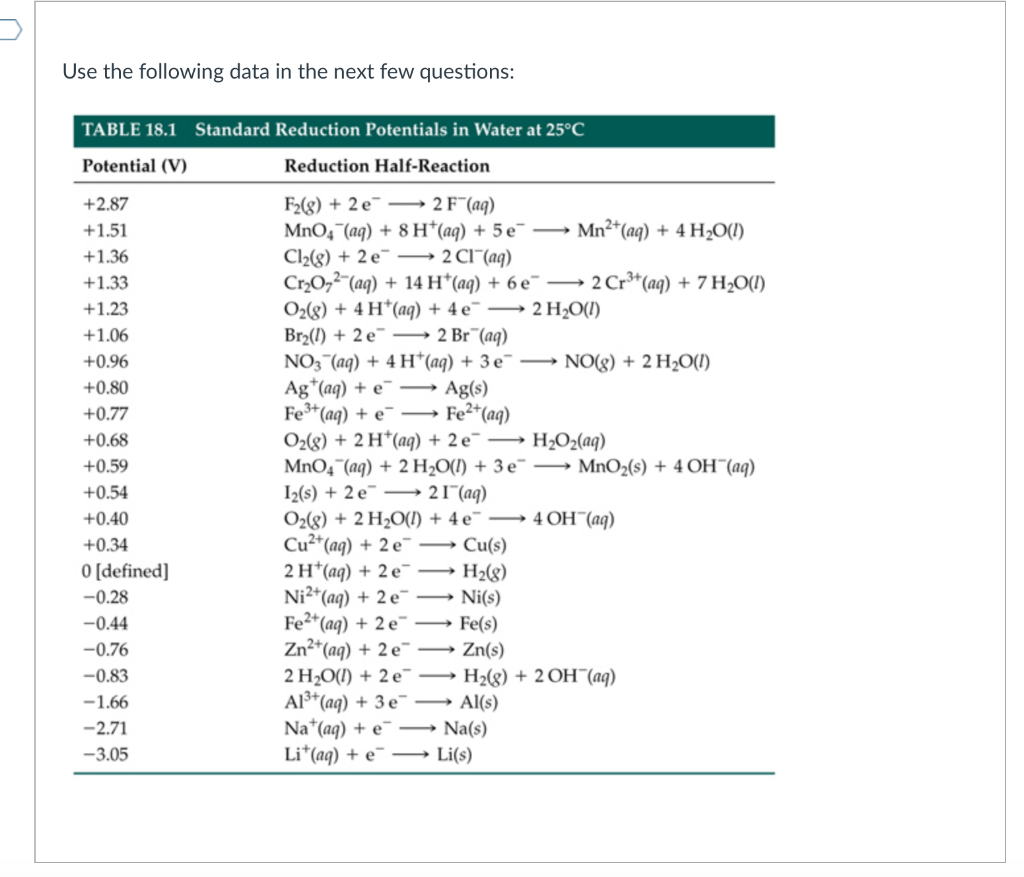
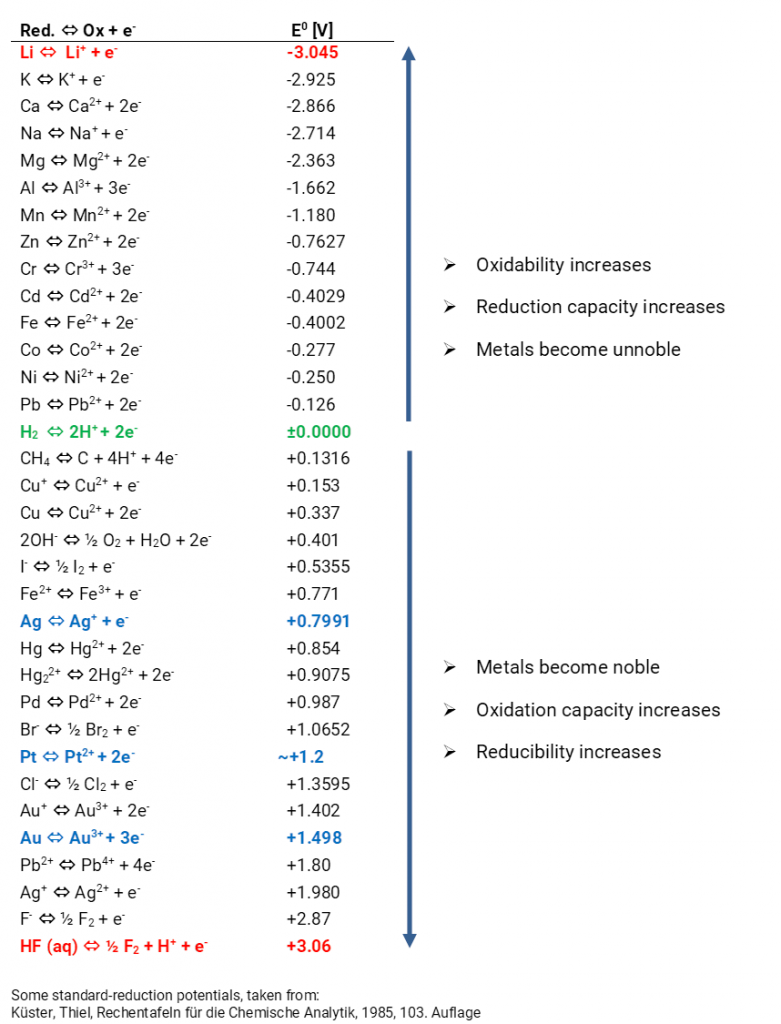
Chemistry - ELECTROCHEMICAL SERIES AND ITS APPLICATION:- A list of elements arranged in order on the basis of their standard reduction potential or oxidation potential is called electrochemical series. EXPLAINATION:- Different elements

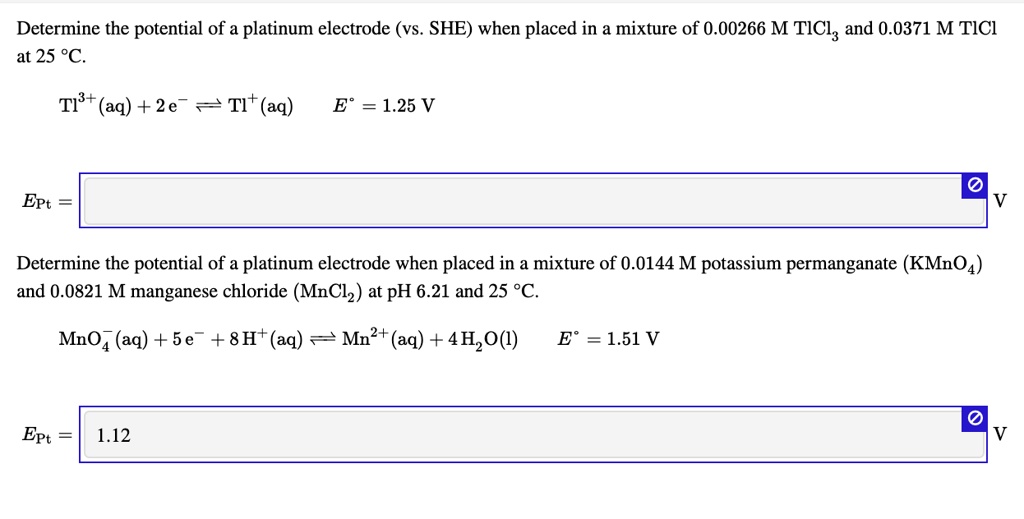
Calculate the reduction potential of a half cell consisting of a platinum electrode immersed in 2.0 MFe^2 and 0.02 M FE^3 solution. Given E^0Fe^3/Fe^+2 = 0.771 V .
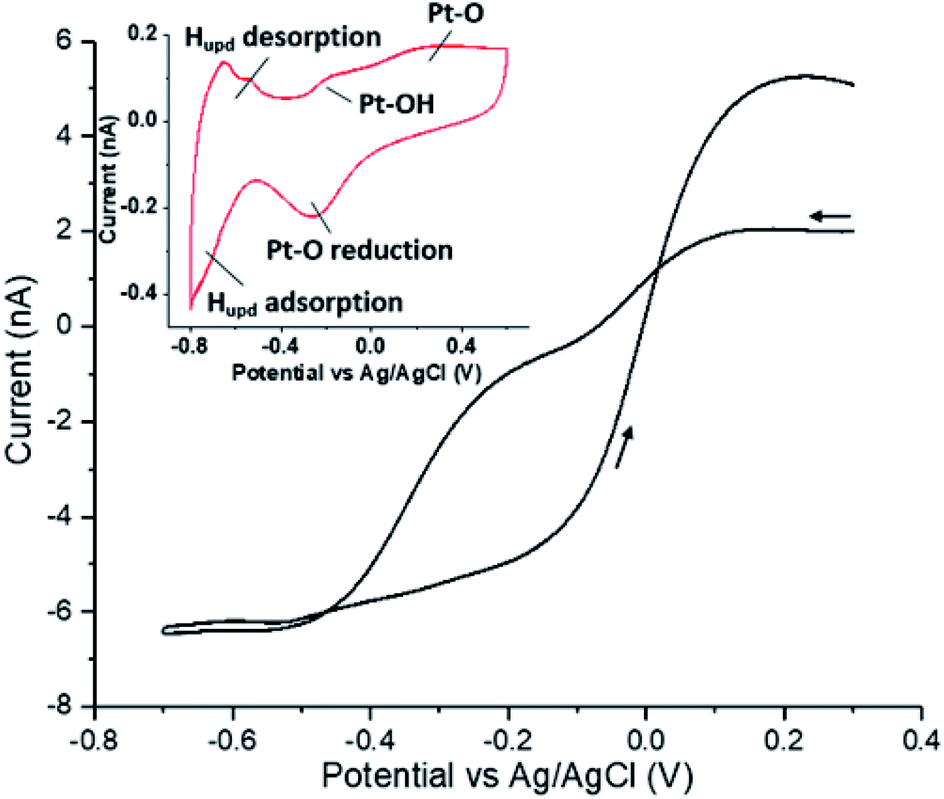
Hydrogen peroxide reduction on single platinum nanoparticles - Chemical Science (RSC Publishing) DOI:10.1039/D0SC00379D