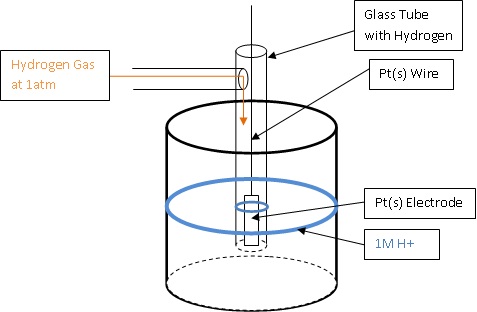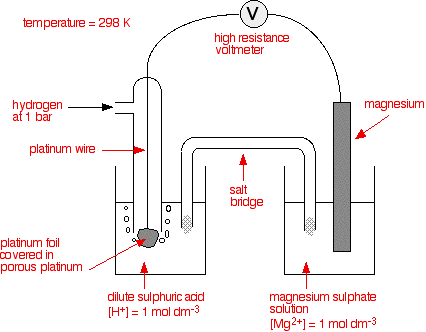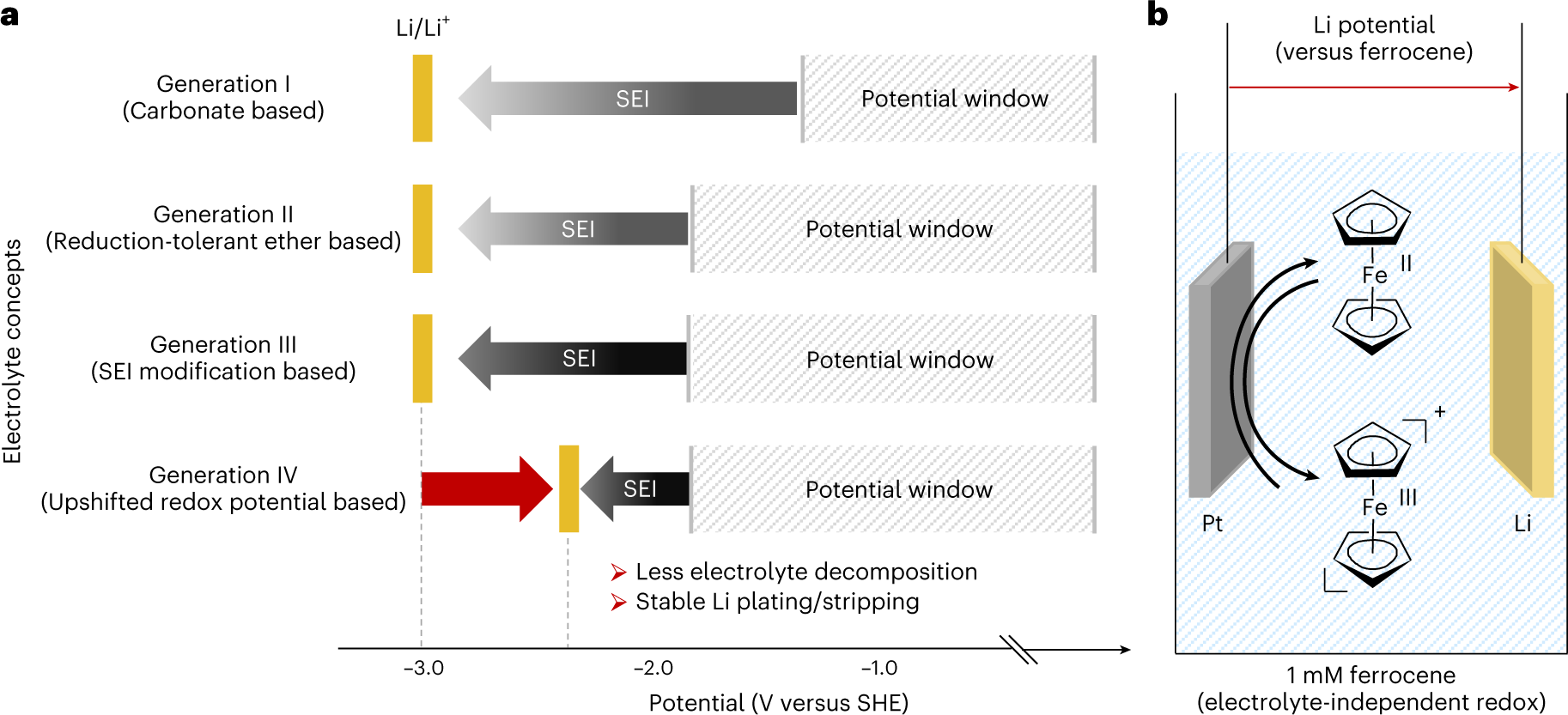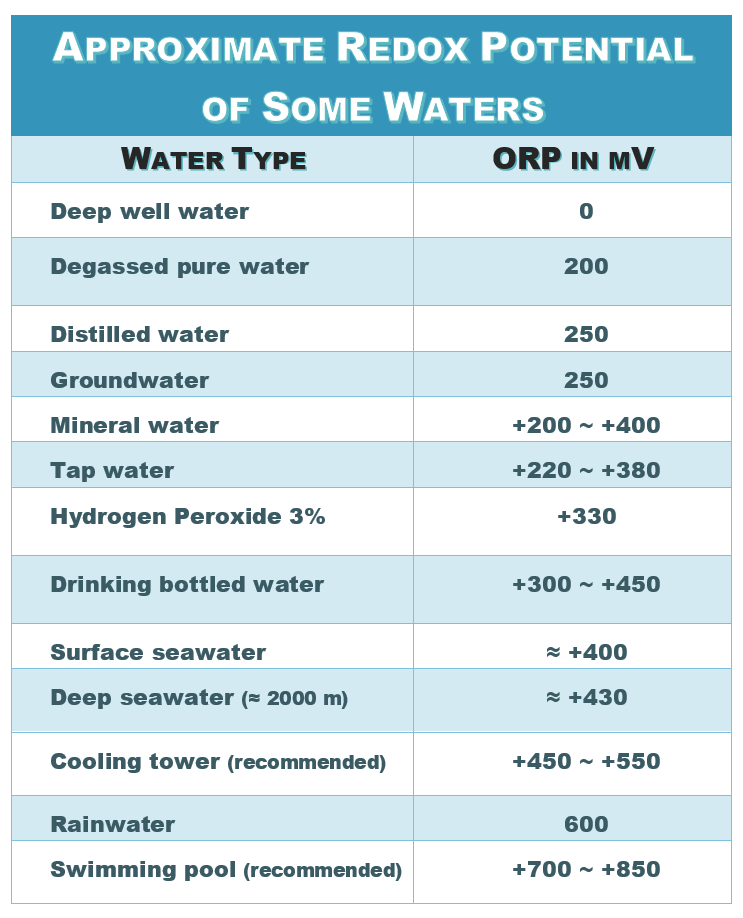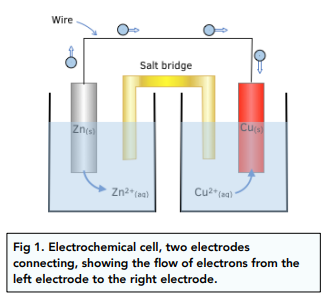
Electrode Potentials and Electrochemical Cells - Electrochemical Cells Reactions (A-Level Chemistry) - Study Mind

Quinone 1 e– and 2 e–/2 H+ Reduction Potentials: Identification and Analysis of Deviations from Systematic Scaling Relationships | Journal of the American Chemical Society
6. The standard electrode potential E^° I2/I , E^° Br /Br2 and E^° Fe/Fe2+ are respectively +0.54V, 1.09V and 0.44V. On the basis of above data which of the following process is

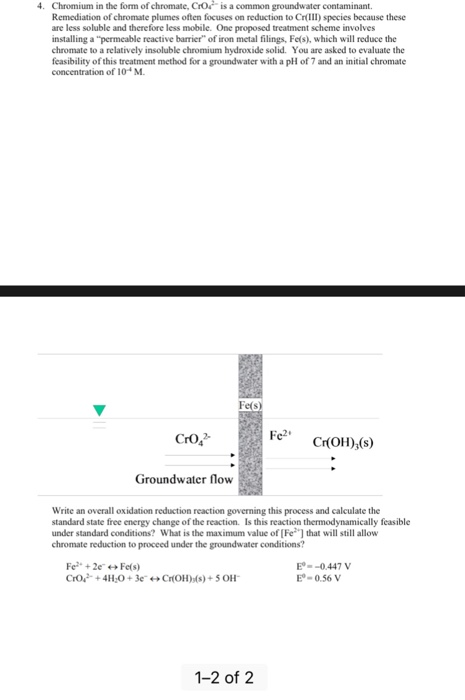
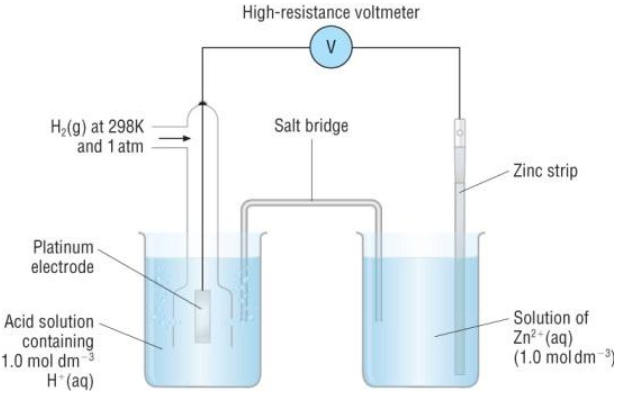




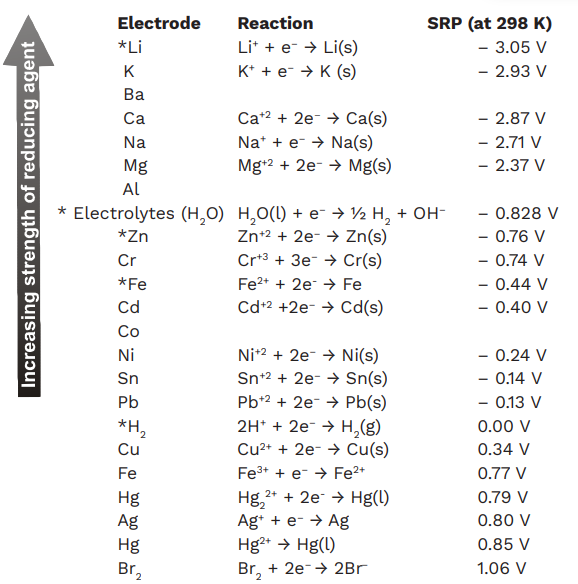

![Electrode potentials [SubsTech] Electrode potentials [SubsTech]](https://www.substech.com/dokuwiki/lib/exe/fetch.php?w=&h=&cache=cache&media=standard_electrode_potential.png)