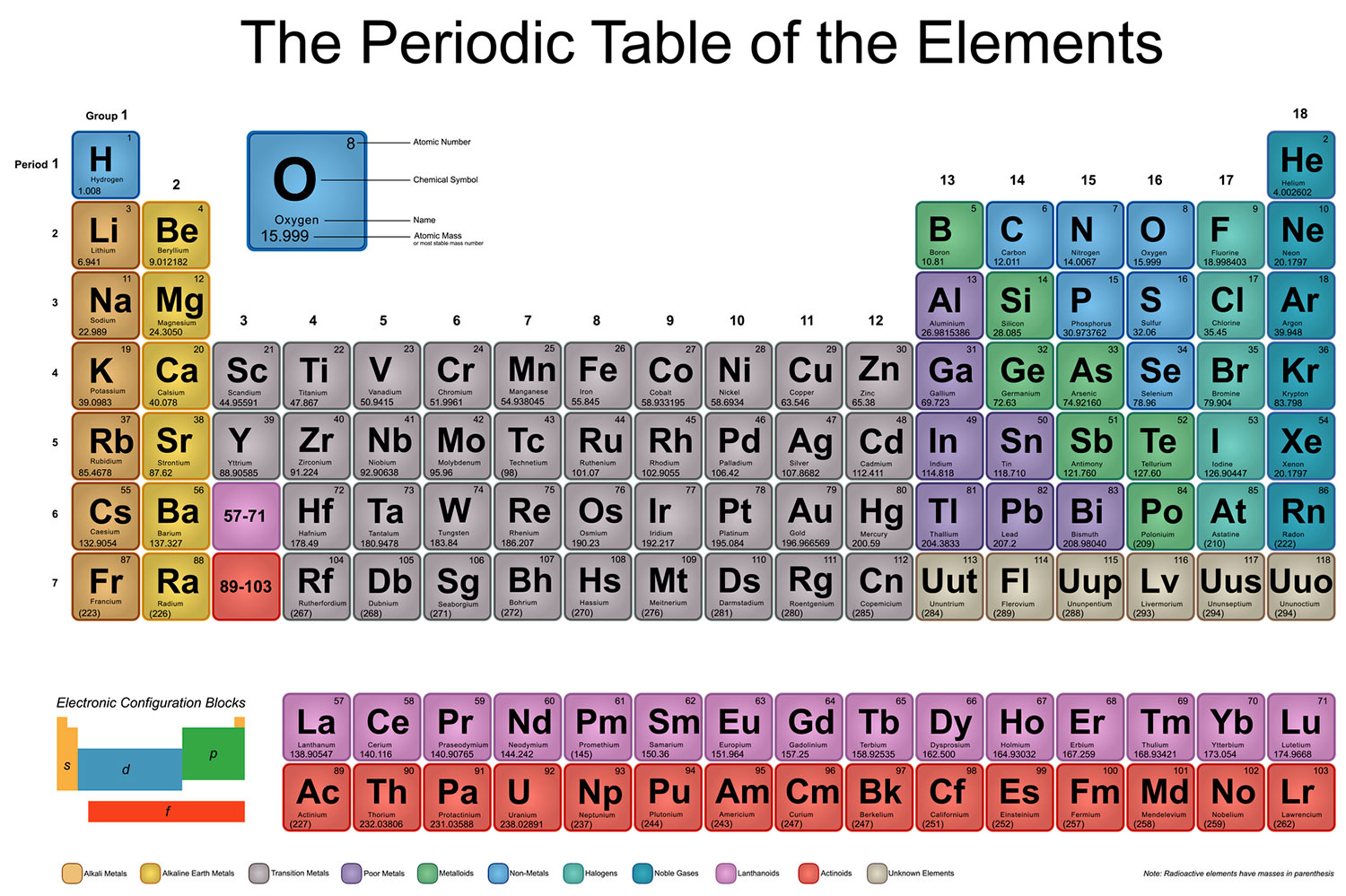
a) why is potassium more reactive than lithium b) why fluorine is more reactive tahn chlorine - Chemistry - Periodic Classification of Elements - 12977123 | Meritnation.com
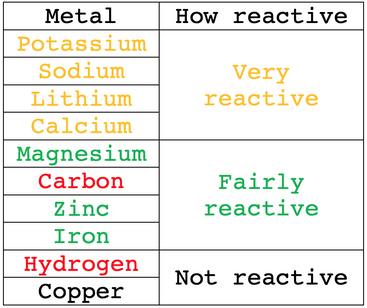
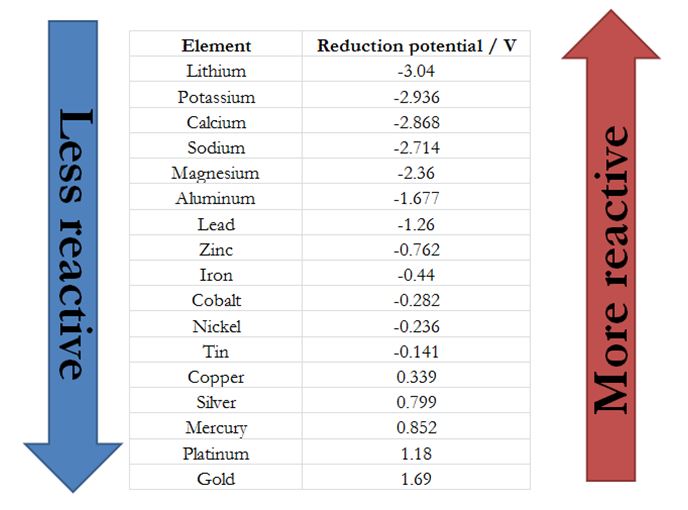
Which element is more reactive among lithium, sodium, potassium, rubidium or caesium? What is their order of reactivity? - Quora

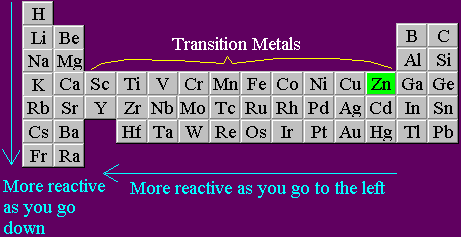
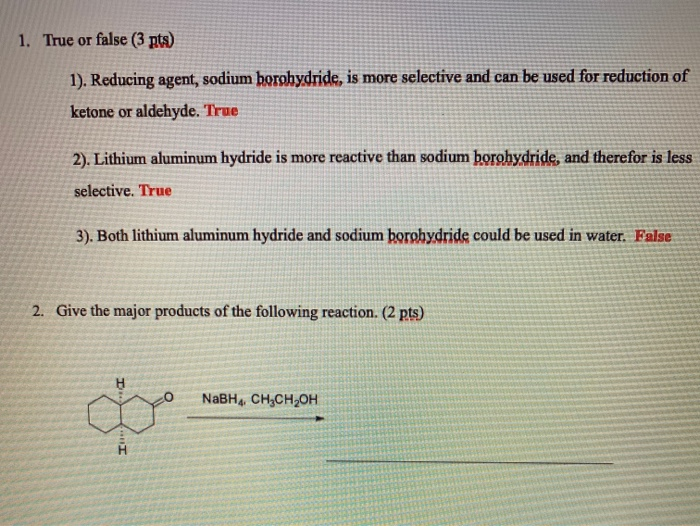
In group 1 (alkali metals), sodium is more reactive than lithium, or lithium is less reactive than sodium. Give reason.

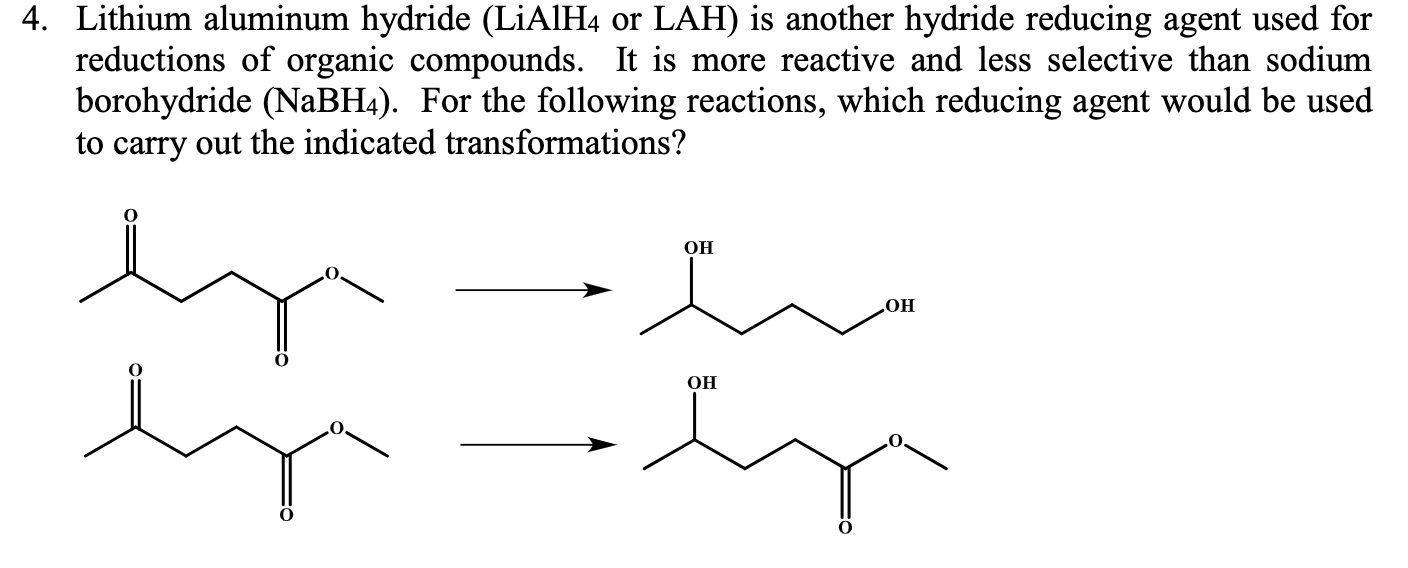
Why the increasing order of reactivity among group one element is Lithium less than sodium less than potassium less than - Chemistry - Classification of Elements and Periodicity in Properties - 11838207 | Meritnation.com